- Sponsored Content
Thirty Years of Monoclonal Antibodies and Protein A: A RetrospectiveThirty Years of Monoclonal Antibodies and Protein A: A Retrospective
January 26, 2018
Sponsored by GE HealthCare Technologies

Photo 1: A page from Herman Waldmann’s notebook
In 1980 at the University of Cambridge’s department of pathology, I worked with Herman Waldmann to develop monoclonal antibodies (MAbs) as treatments for graft-versus-host disease (GVHD). That disease is associated with serious complications of stem cell transplantation when attacking T-cells can damage the lungs, liver, skin, and other organs. If we could find a specific MAb that would work with the human complement system to kill those cells, they could be selectively removed from the bone marrow. The human complement system enables the body to destroy cells targeted with antibodies.
The First Monoclonal Antibodies
From a great collection of rat monoclonal antibodies of unknown specificity (Photo 1), we selected antibodies that could kill T-cells with human complements. Because we wanted to use immunoprecipitation to purify those antibodies, we further screened selected molecules for protein A binding. The selected antibody was named Campath after the Cambridge Pathology Department.
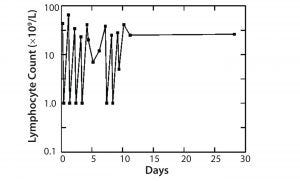
Figure 1: Treatment of leukemia with Campath-1M
The first MAb, Campath-1 IgM antibody, depleted T-cells in the blood of a patient with leukemia to <1% of starting level. Although those cells returned the next day, we could show that the treatment was fairly safe, that T-cells could be attacked, and that complement could be activated. When the same treatment was conducted with bone marrow, we found >99% of T-cells were removed from the bone marrow. When patients who needed the transplant with this bone marrow were infused, their instance of GVHD was greatly reduced (Figure 1).
However, manufacturing Campath-1 presented some problems. Although the antibody would bind to protein A, the low pH needed to elute it damaged the IgM molecule. In addition, the patient rejected some bone marrow grafts. Normally, because a recipient is immunosuppressed, T-cells from a donor get the upper hand, thereby causing GVHD (because there are a lot of T-cells). After removing those cells, the T-cells that remain in the recipient can attack the graft and cause graft rejection. Graft rejection had been seen in about 10% of patients.
In mouse models, Fc receptors on killer cells needed to be engaged to provide a way of destroying the T-cells and prevent graft rejection. Hence, we looked for an antibody the same property in humans. The only isotype of rat antibodies that would bind with Fc receptors and engage killer cells was rat gamma 2b. Using hemagglutination, we screened a large numbers of cells to obtain a rat gamma 2b against the Campath-1 antigen.
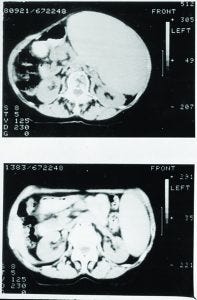
Figure 2: Treatment of non-Hodgkin’s lymphoma (NHL) with Campath-1H
The Campath-1G antibody was highly effective in preventing graft rejection. When tested in a patient with leukemia and previous unsuccessful treatment with Campath-1M, even small doses depleted T-cells, and the numbers stayed low for quite a long time. In this case, treatments were discontinued when the patient developed an antibody response against the rat antibody. However, Campath-1G was highly effective for preventing graft rejection in leukemia patients because it was given for only a short period at the time of the transplant. For other disease types (e.g., autoimmune diseases such as arthritis or multiple sclerosis) for which repeated doses could be needed, the antibodies would need to be humanized.
Eventually, Campath-1G was humanized, and the resulting Campath-1H progressed into the clinic from our laboratory. In a patient with end-stage lymphoma, in whom lymphoma cells had spilled out into the blood (similar to leukemia), Campath-1H depleted those cells effectively (Figure 2).
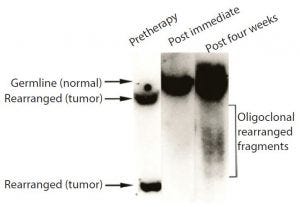
Figure 3: Samples of bone marrow taken before treatment, probed for the characteristic gene of the tumor cells; before treatment, sample contained only tumor cells. By contrast, none of the tumor cell DNA could be detected in a sample taken shortly after Campath treatment.
When we evaluated the recovering cells (which came back after more than a month), they were healthy (Figure 3). We observed same with the bone marrow and no signs of tumor cells, as was the case with Campath-1M and Campath- 1G.
Until that point, we had conducted our work in an ordinary research laboratory. To scale up the production, we needed a better manufacturing facility with improved quality control. So the first good manufacturing practice (GMP) facility in the United Kingdom to be embedded in a university (an academic facility) was built. With increasing antibody titers, the use of hollow-fiber fermentors, and a need to work within a small budget, we had to reuse protein A resin by cleaning and sanitizing to determine whether protein A could withstand sodium hydroxide, and we investigated different brands of protein A resins.
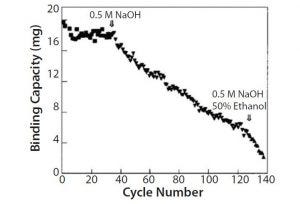
Figure 4: Conventional chromatography compared with membrane adsorbers
From those results, we selected protein A Sepharose™ Fast Flow resin. It could be treated with sodium hydroxide at 0.5 M (which was what we had been using for sanitizing our columns) for up to 40 cycles in pilot production scale (Figure 4).
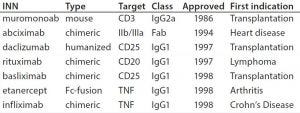
Table 1: Some of the first approved chimeric and humanized antibodies
Increasing Number of Monoclonal Antibody Approvals
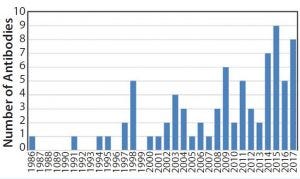
Figure 5: Approval of antibody products 1986–2017
By the 1990s, we had taken about 20 different MAbs from our research laboratory into the clinic. In 1997 and 1998, several chimeric and the first humanized antibodies were approved (Table 1). Campath-1H was approved in 2001, shortly followed by Humira™ (adalimumab). So far, the year 2017 appears to be setting a record in MAb approvals, with about eight approvals so far and perhaps more by year end (Figure 5).
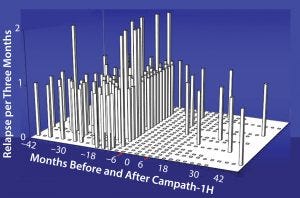
Figure 6: Relapse rate in 22 patients with relapsing-remitting multiple sclerosis; there was a 91% reduction in relapse rate after Campath. Before treatment there were 2.21 relapses/
patient/year. After treatment there were only 10 investigator confirmed episodes, 0.19 relapses/patient/year.
Studies have shown for some time that Campath-1H could be used for treating multiple sclerosis (Figure 6). The treatment has been over a course of only five days, followed by another five-day treatment one year later. During treatment, which depletes lymphocytes and powerfully immunosuppresses patients, the instances of adverse clinical events were greatly reduced. In addition, the patients’ levels of disability seemed to improve (Figure 7). Results from a follow-up study show that clinical improvement was maintained 10 years later.
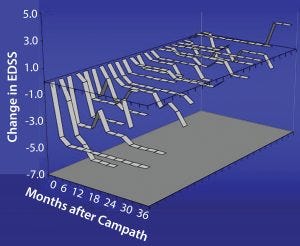
Figure 7: Change in disability after Campath for relapsing remitting group, which showed a decrease in EDSS (less disability with time); damage to axons is not so severe, and there may be repair of the myelin sheath with consequent
improvement in function.
Campath antibodies recognize an epitope very close to the cell membrane (Figure 8). The proximity of that epitope to the cell membrane is one of the reasons why Campath is such a good antibody for killing cells. The antibody delivers that lytic target only to places where it is needed (close to the cell membrane). Other antibodies that are effective at killing cells almost always tend to bind closely to the cell membrane.
Monoclonal Antibody Purification
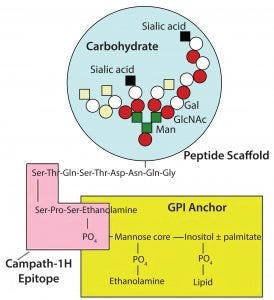
Figure 8: Campath antibodies recognize an epitope very close to the cell membrane
We developed a method for purifying Campath antibodies using the target molecule as an affinity ligand (Figure 9). This approach had the advantage of requiring only very weak acid for elution. However, this strategy was never implemented because protein A chromatography was already well established, and the lower pH needed to elute antibodies from protein A has some advantages for virus inactivation. Several other alternatives also have been investigated, but today protein A chromatography remains useful.
A 2016 publication summarizes about 40 years of improvements made in protein A chromatography used in the therapeutic antibody industry (1). During that period, big improvements were made in the productivity of cell lines. At first, we could produce only micrograms of antibody per milliliter of culture. Today, you can get up to multimilligrams quite easily. Even bigger increases in protein A column capacity have taken place as well as the columns’ ability to withstand harsh cleaning agents such as sodium hydroxide.
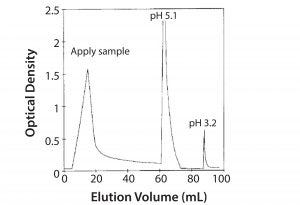
Figure 9: Affinity purification of Campath on mimotope peptide
Some researchers have discussed whether protein A will be displaced or remain a part of the bioindustry for many more years. Antibody therapeutics have been around now for quite a long time, and most people believe they will continue to be around for a lot longer.
One area of interest and investment is the development of biosimilar antibodies. With pipelines containing hundreds of innovator and biosimilar antibodies, I hope and believe there will be many exciting therapies to come. I see little evidence that protein A chromatography will be displaced.
With all the investments in improving this technology, immunoglobulin and protein A are a partnership: One is being used for the purification of the other to make many more powerful and effective drugs for the future.
Learn more: 
References
1 Bolton GR, Mehta KK. The Role of More than 40 Years of Improvement in Protein A Chromatography in the Growth of the Therapeutic Antibody Industry. Biotechnol. Prog. 32(5) 2016: 1193-1202. •
Formerly a visiting professor of therapeutic immunology at the University of Oxford (Oxford, UK), Geoff Hale is Managing Director at BioArchitech Ltd. in Cambridge, UK.
You May Also Like





