- Sponsored Content
- Gene Therapies
- Personalized Medicine
Transforming Personalized Medicine into Off-the-Shelf Cell TherapiesTransforming Personalized Medicine into Off-the-Shelf Cell Therapies
May 24, 2020
Sponsored by Mogrify
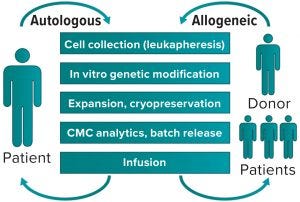
Figure 1: Autologous (left) and allogeneic (right) generation of cell therapy products
Initial progress in cell and gene therapy has seen 12 advanced therapeutic medicinal products (ATMPs) become available on the market in 2019 for a range of conditions, from monogenic diseases to cancer. Despite such progress, development of clinically and commercially successful cell therapies presents manufacturability challenges and questions about bypassing patients’ immune systems. The availability of rapid sequencing and next-generation bioinformatics has made it possible to understand the mechanisms of disease better and accelerate development of therapeutic responses. The same toolbox will enable our fight against cancer (with advanced immunotherapies) and other conditions.
The Autologous Approach
In cell therapy for immunooncology, often the medicinal product is a cell that has acquired a therapeutic function through genetic modification induced by a virus. That is achieved by collecting relevant cells from donors, then genetically modifying and expanding them in culture to obtain the numbers required for infusion into a recipient. Most often, that has been an autologous strategy in which donor and recipient would be the same person — a patient (Figure 1, left). Such a vein-to-vein approach presents some difficulties because chemistry, manufacturing, and control (CMC) activities all have to be completed under extremely aggressive timelines (e.g., two to three weeks) dictated by protocols that patients undertake as part of their therapy. Those protocols are designed both to maximize collection of starting material that will become the medicinal product and to provide the best engrafting conditions at the time of infusion.
Autologous therapies are prime examples of personalized medicine. Making one medicine for treating one person’s condition builds on decades of experience gained in the field of bone marrow transplantation and has been adopted in the field of immuno-oncology. Two autologous products — Yescarta (axicabtagene ciloleucel) from Kite Pharma and Gilead Sciences and Kymriah (tisagenlecleucel) from Novartis — have been approved for commercialization and are now on the market. Both products are designed to empower T-cells with a chimeric antigen receptor (CAR) to recognize and kill cells that express the cluster of differentiation (CD)19 protein on their surfaces. CD19 is found on B cells, particularly malignant B cells in some forms of hematological malignancies. Both of those products have elicited good overall responses followed by durable results in many patients.
Even though autologous therapies have the advantage of eliminating concerns related to graft-versus-host disease, they are inherently expensive. They carry high costs and variability associated with an ad-hoc manufactured product. For each single-dose batch, not only will the quality and quantity of the starting material be different — thus requiring adjustments to downstream processing each time — but the final product itself also will be different depending on efficiency of viral transduction as well as purities and potencies achieved. Each such batch released will have to be qualified by a certificate of analysis (CoA), often comprising a matrix of analytical test results to ensure safety, identity, and potency of the product.
Those are nontrivial tasks for companies trying to turn around hundreds to thousands of batches per year, and as such, they have resulted in substantial costs per treatment. Because cell therapy is a technology that is still in its infancy, we can expect associated costs to drop over time, even when providing required manufacturing “at the bedside.” To that end, combining the scaling-out of manufacturing with closed systems, automation, and process simplification with increased accessibility and affordability of manufacturing suites (already available at several hospital sites) will reduce the costs of autologous products provided in a distributed strategy, as required.
The Allogeneic Approach
That raises the question of whether an alternative approach can be considered. The body of knowledge developed so far from current autologous cell therapies could represent a solid basis on which to build the next range of products. These new products could advance the concept of personalized medicine by optimizing and hybridizing the autologous model or offering a single allogeneic cell therapy product for all patients.
From a manufacturing perspective, such products will share many of the challenges with traditional biologics: efficacy, safety, and scalability. The ultimate goal would be to scale up manufacturing based on a centralized model to produce affordable batches for hundreds of patients — all quality controlled, stored, and ready for use — and thus generate “off-the-shelf” products.
To achieve that, the scientific community has focused attention on identifying the best allogeneic cell therapy system, in which cell donors and recipients are not the same people (Figure 1, right) This should provide unlimited amounts of identical starting materials that, after genetic modification, will engraft safely in all patients to exert expected functions. But do such donors exist? In cell therapy products for immunooncology — e.g., CAR T-cells — it is likely that recruiting healthy individuals as donors will provide higher yields and perhaps better-quality starting materials than are possible in an autologous strategy.
Using T cells obtained by leukapheresis as a starting material probably will be sufficient to manufacture enough finished product for a few histocompatible patients at least. That is a step toward development of more affordable products, but it remains a laborious manufacturing process for a limited number of patients.
An alternative to obtaining cells by leukapheresis is using well-characterized induced pluripotent stem cells (iPSCs). Through systematic optimization of a dedifferentiation protocol to eliminate product-related and process-related impurities while reducing the timelines, generation of iPSCs can be scaled up to provide large amounts of starting material for thousands of patients. In turn, that could enable large, fully characterized and validated cell banks for the most common haplotypes to be generated, then subsequently used to manufacture different cells of interest in fully automated and cost-effective processes. Establishing and banking a “universal” iPSC line, offering compatibility to all haplotypes, could bring the goal of developing a true “off-the-shelf” cell therapy product a step closer to reality. Several attempts to do so are under way and have generated great interest in immunooncology. A further scalable source of starting material could be generated through cell transdifferentiation and systematic, data-led conversion technologies to enable considering any cell type as a source of starting material for downstream cell-therapy processing.
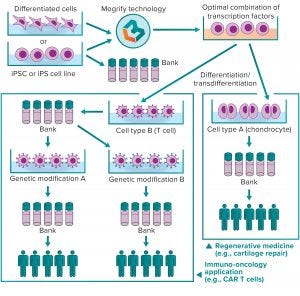
Figure 2: Mogrify cell-conversion technology for cell therapy
The Next Step
Optimizing and scaling up good manufacturing practice (GMP)-compliant differentiation protocols will be vital to creating safe and efficacious cell therapeutic products from iPSCs. Streamlining those processes undoubtedly will be guided by data-driven computational approaches and manipulation of large-scale data. Mogrify Ltd. has developed a data-driven bioinformatic approach that will play a key role in the future of cell therapy development and manufacturing. Using next-generation sequencing and gene-regulatory information to identify how to convert any human cell into any other with transcription factors (TFs) or small molecules, this technology provides an elegant method for systematic cell programming and reprogramming. Although their differentiation capacity has made iPSCs many scientists’ starting cell type of choice, Mogrify technology allows researchers to start with any cell type and induce transdifferentiation into immune cells needed for therapy. A propriety algorithm identifies an optimal set of TFs and culture conditions for doing so. Those are delivered by existing methods (e.g., retroviruses and adenoassociated viruses) to differentiate large quantities of a desired cell type with high specificity, shorter timelines, and lower costs. Therefore, the technology could be used to generate large GMP-compliant cell banks ready for further downstream manipulation (Figure 2).
In recent years, cell therapies finally have entered the healthcare market, generating a first wave of immunooncology products that offer new hope for patients. A revolution in cell therapy is upon us now, with laboratories across the world already designing the next generation of products. That new generation will have increased efficacy, improved safety, and expanded scalability — allowing for affordable cell therapy products to treat new indications such as autoimmune and infectious diseases.
Dr. Alessandra De Riva is director of development at Mogrify Ltd., 25 Cambridge Science Park, Cambridge, CB4 0FW, United Kingdom; 44-1223-734154. Mogrify is a registered trademark.
You May Also Like





