- Sponsored Content
Use of Dynamic Control in Periodic Counter-Current ChromatographyUse of Dynamic Control in Periodic Counter-Current Chromatography
August 10, 2016
Sponsored by GE HealthCare Technologies
 Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTA™ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors for loading (Figure 1). This study shows how the dynamic control functionality of ÄKTA pcc 75 can be used to adjust for variations in binding capacity of chromatography resin or for changes in feed composition when, for example, perfusion cell culture is being used. In this study, dynamic control was used for a protein A monoclonal antibody (MAb) capture step operated in a three-column PCC (3C PCC) setup.
Interest in continuous bioprocessing is growing rapidly, and such an approach has shown to have a potential in providing gains in both productivity and savings in cost of goods sold. The ÄKTA™ pcc 75 chromatography system can be used for purification of target proteins in continuous downstream processes using periodic counter-current chromatography (PCC). PCC enables greater use of chromatography resin capacity, allowing sample loading to much higher levels than what is possible in traditional batch chromatography by using safety factors for loading (Figure 1). This study shows how the dynamic control functionality of ÄKTA pcc 75 can be used to adjust for variations in binding capacity of chromatography resin or for changes in feed composition when, for example, perfusion cell culture is being used. In this study, dynamic control was used for a protein A monoclonal antibody (MAb) capture step operated in a three-column PCC (3C PCC) setup.
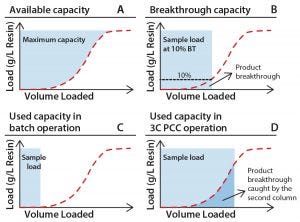
Figure 1: Capacity use for standard and PCC; (A) total available capacity of a chromatography resin; (B) capacity typically measured during process development experiments; (C) capacity typically used in manufacturing after adding safety factors; (D) example of capacity utilized when implementing PCC. Note that product breakthrough is captured by the next column in the loading zone.
Variable Feed Concentration
To show the system’s ability to adjust for changes in feed concentration, two different 3C PCC cases were considered:
continuous change in feed concentration
alternation between two feed concentrations.
The experiments used HiTrap™ MabSelect SuRe™ LX, 5-mL columns. The dynamic control function of ÄKTA pcc 75 was able to adjust for differences in feed concentration. As can be seen for the breakthrough signal (blue) in Figure 2 (top), the time, UV absorbance level, and steepness of the UV curve change as the MAb concentration is changed between 1.8 mg/mL to 1.5 mg/mL.
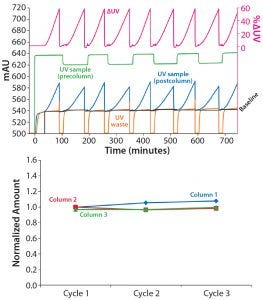
Figure 2: (top) Switching between MAb concentrations of 1.5 and 1.8 g MAb/L during load onto MabSelect SuRe LX at 2.5 min residence time; (bottom) amount of MAb eluted per column (Col 1 to Col 3) when switching between feed concentrations of 1.5 and 1.8 g MAb/L during load onto MabSelect SuRe LX at 2.5 min residence time
Still, breakthrough is consistently maintained at the defined ΔUV (pink) by the dynamic control functionality, and the difference in concentration is reflected only in the time before the breakthrough occurs. Consistency in sample load can be seen in the low variation in the amount of eluted MAb, with relative variations of <3% (Figure 2, bottom).
As can be seen for the breakthrough signal (blue) in Figure 3 (top), a similar pattern can be observed when using a MAb concentration gradient from 1 g/mL to 2 g/mL. The time, UV absorbance level, and steepness of the UV curve closely follow the changes in MAb concentration, and breakthrough is consistently maintained at the defined ΔUV (pink). Also in this case, the variation in the amount of eluted MAb is low, with relative variations of <3% (Figure 3, bottom).
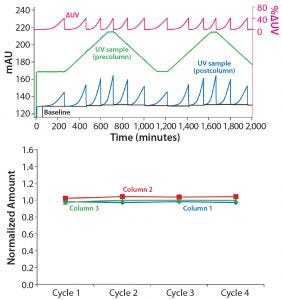
Figure 3: (top) Dynamic control when using a feed concentration gradient from 1 to 2 g MAb/L during load onto 5 mL HiTrap MabSelect SuRe LX columns at a 3.5 min residence time; (bottom) amount eluted MAb per column (Col 1 to Col 3) when using a gradient from 1 to 2 g MAb/L during load onto MabSelect SuRe LX resin at a 2.5 min residence time
Variable Resin Capacity
A decrease in resin binding capacity can occur over time. Variations in resin binding capacity also can be due to differences in resin volume between packed column beds. MabSelect SuRe chromatography resin has lower binding capacity than MabSelect SuRe LX resin and was used here to mimic such a situation. To show the system’s ability to adjust for changes in column performance, dynamic control was used to control a 3C PCC setup on ÄKTA pcc 75 equipped with one MabSelect SuRe column (Col 1) and two MabSelect SuRe LX columns (Col 2 and Col 3) (Figure 4).
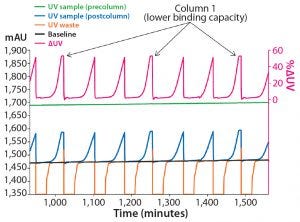
Figure 4: Difference in DBC between MabSelect SuRe (Col 1) and MabSelect SuRe LX (Col 2 and Col3) resins at 5 min residence time for a MAb concentration of 4.5 g/L; MabSelect SuRe reaches the set ΔUV faster than MabSelect SuRe LX, shown as the ΔUV signal plateau occurring every third load (arrows).
The dynamic control function of the ÄKTA pcc 75 system detects the lower dynamic binding capacity (DBC) of Col 1 (MabSelect SuRe resin) as the breakthrough occurs earlier on this column. In this case, the method was designed to halt the load until a new column is ready to be added to the loading zone. The halt in loading can be seen as a different peak shape in the chromatogram because there is no change in UV during the halt and the UV peaks flatten out. Those peaks, representing the breakthrough of the MabSelect SuRe column, are marked by arrows in the chromatogram. The lower DBC of the MabSelect SuRe resin is reflected in the amount of eluted antibody from this column during each cycle. Despite the difference in DBC between the resins, the relative variance with regard to the amount eluted MAb per column was <3%. The observed consistency in performance was enabled by the dynamic control function and would not have been achieved if the system instead had been run based on predefined column switching times.
Conclusions
The use of the dynamic control function enabled operation of the ÄKTA pcc 75 system under process conditions, at which either the feed concentration or the chromatography resin capacity was changed. If static control with fixed load times had been used, an increase in MAb concentration or decrease in resin binding capacity would have resulted in product loss. With dynamic control, on the other hand, differences in resin performance were detected and adjusted for. The ability of dynamic control to adjust for variations in feed concentration makes ÄKTA pcc 75 well-suited for processing of perfusion cell culture feeds, where changes in the feed composition can occur. A more detailed description of this work is given in application note 29169950 AA (1).
Reference
1 The Use of Dynamic Control in Periodic Counter-Current Chromatography. GE Healthcare, 29169950, Edition AA, 2016.
Hans Blom is a scientist at GE Healthcare Bio-Sciences AB, Björkgatan 30, 751 84 Uppsala, Sweden. GE, GE monogram, ÄKTA, HiTrap, and MabSelect SuRe are trademarks of General Electric Company. ©2016 General Electric Company. First published July 2016.
You May Also Like





