Clinical Supply Chain: A Four-Dimensional MissionClinical Supply Chain: A Four-Dimensional Mission
March 11, 2016
A clinical supply chain fulfills perfectly all four characteristics of what Packowski describes as a “VUCA” (volatility, uncertainty, complexity, and ambiguity) world (1). In commercial markets, supply chains depend predominantly on consumer orders. For global drug development programs, both investigators and patients can be considered end consumers.
The international journey of a specific investigational medicinal product (IMP) includes all of the following: global sourcing of comparators, manufacturing, storage, distribution, site/patient (consumer) management, and return and destruction of the IMP. Application materials (e.g., infusion pumps and some medicinal products and biological samples) may need to be stored in refrigerated conditions.
In most clinical studies, the majority of data is analyzed using patients’ laboratory samples. Those samples are collected in syringes, blood-collection tubes, urine-specimen containers, and so on. Patient blood samples drawn in special tubes may need to be centrifuged and distributed to analytical laboratories in cooled conditions. Myriad other supplies need to be on hand to conduct clinical trials. For example, documents such as trial protocols, study-specific patient information, and paper patient diaries also are commonly distributed.
Currently, the biopharmaceutical industry is including a growing number of mobile measurement devices designed to analyze patients’ conditions, support trial efficacies, and monitor safety. Given the many variables present in highly complex and uncertain clinical trial environments, a holistic approach is necessary to manage uncertainties along a clinical supply chain journey.
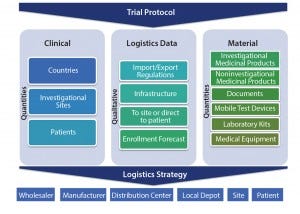
Figure 1: Clinical trial logistics, planning aspects
Integrated Trial Planning
A demand for clinical trial materials is created by trial design. Supply chain planning starts with integrated trial protocol development, review, and optimization before finalization. Clinical, statistical, and medical experts typically integrate central laboratory providers and contract manufacturing organizations (CMOs) during early planning. Furthermore, when supply chain experts are involved early on, they can rapidly
analyze necessary drivers connected with supply chain complexity. Such complexities can be categorized and understood using highly simplified models that show three major aspects: clinical drivers, logistics information, and materials needed (Figure 1).
Clinical: The required number of patients can be used to determine how many sites will be necessary and help researchers specify the countries needed to reach enrollment targets. These variables create a significant level of ambiguity for a supply chain.
Logistics Data: Qualitative information assists in reducing uncertainty associated with study planning. Factors such as import and export regulations as well as local and on-site logistics infrastructure may exclude certain countries or hospitals from the selection process. Logistics data supports a solid feasibility process and creates a more accurate patient enrollment forecast.
Material: A trial protocol significantly influences required types and the number of materials. Researchers should assess which laboratory analyses are necessary. And they should determine whether the frequency of testing intervals can be reduced or whether they can run analyses on only subpopulations. It is well known that patient engagement is key to achieving high levels of drug adherence. Mobile devices can support increased sensitivity (e.g., frequent home testing of clinical parameters and electronic patient-reported outcome (ePRO) diaries). Considering the many variables that can alter a clinical trial protocol, what is necessary to achieve the best trial results may not necessarily always be the high-end solution.
Logistics Strategy: A rock-solid clinical trial design will inevitably lead to a holistic, end-to-end logistics strategy. Each trial will require a specific and modified logistics approach, but the chain of custody must remain the same in principle. “Siloed” operations between various supply-chain stakeholders increase risks and typically create unnecessary additional costs.
When biopharmaceutical research and development companies closely integrate key supply chain providers into planning, execution, and technical and quality perspectives, those companies tend to be much more successful. Those providers can be CMOs, central laboratories and logistics providers, and/or clinical trial–conducting clinical research organizations (CROs). By contrast with other industries, many biopharmaceutical clinical supply chain providers are still miles away from fast-moving product cycles of today’s market.
Risk Management: To simplify clinical supply-chain planning, numerous risks must be quantified, and corresponding mitigating measures must be put in place. Risk management is essentially important when defining a final logistics strategy because many adverse events will directly influence a supply chain. In a worst case, such events can even lead to clinical-trial disruption.
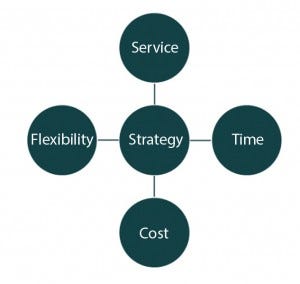
Figure 2: Align supply chain strategy
Service, Time, Cost, and Flexibility Battle
Logistics experts are aware of the difficulty in achieving timely trial delivery with balanced cost. The constant battle between service, time, cost, and flexibility makes standardization of services a challenge (Figure 2). If needed, almost any product in the world can be transported within 48 hours from a manufacturer or a depot to any investigator location in the world, but certainly that would be at extreme expense.
So manufacturers must balance the four dimensions of volatility, uncertainty, complexity and ambiguity (1). At the beginning of a single supply chain project, all key stakeholders in a clinical drug development program should understand clearly the balance of those four dimensions. Although the time of delivery is a key factor (especially for patients with urgent needs), all four dimensions also can be influential. Facilities should carefully assess whether product delivery to a site needs to happen overnight. With upfront planning, automated end-toend stock management control, use of randomization and trial supply management (RTSM), and enterprise supply chain systems, not every product would need overnight delivery.
For instance, initial supplies may need to be transported by air if a low volume of product is available. But after that initial supply, replenished supplies could be routed by ground transport. Although that solution creates a longer transportation time, leveraging site stock alerts would allow preparation for advanced deliveries.
Consider another example of balancing service, time, cost, and flexibility: When ordering low-price products with long shelf lives in high quantities, a single standardized process can be put in place so that all depots and sites receive a higher overage. That allows sites to eliminate frequent reorders. In such a case, product costs are more than offset by distribution costs. And flexibility remains to expedite emergency shipments in rare cases when required.
A Comprehensive Strategy
The clinical trial supply business is clearly a VUCA (volatility, uncertainty, complexity and ambiguity) world. Clinical information, logistics data, and material information build the model, driven by individual trial protocols. Those quantitative and qualitative parameters must be considered to develop and implement optimal logistics strategies. The four dimensions of services, time, cost, and flexibility must be balanced and may be supplemented by supply strategies implemented for a single project.
Reference
1 Packowski J. LEAN Supply Chain Planning: The New Supply Chain Management Paradigm for Process Industries to Master Today’s VUCA World. CRC Press: Boca Raton, FL, 2013.
Jens Mattuschka is vice president of strategic development, clinical trial supplies and logistics, at PAREXEL, 195 West Street, Waltham, MA 02451; 1-781-487-9900; [email protected]; www.parexel.com.
You May Also Like





