Future Supply-Chain Needs for Allogeneic Cell Therapies: Why Strategic Partnerships Are CriticalFuture Supply-Chain Needs for Allogeneic Cell Therapies: Why Strategic Partnerships Are Critical
Allogeneic products are an attractive option for cell-therapy developers because multiple batches can be manufactured using apheresis material collected from one healthy donor — and because the resulting therapies could be made available as off-the-shelf products. The appeal of this approach is apparent from growth in allogeneic-therapy development. According to the Alliance for Regenerative Medicine, the number of clinical trials for allogeneic cell-based cancer treatments has increased by 30% over the past five years. Early in 2022, allogeneic candidates accounted for 27% of all cell-based immunotherapies under clinical evaluation (1).
However, building a stable, reliable supply chain for cellular starting material is complex. Many processes must occur before donor material can be shipped to a biomanufacturer. Each step raises obstacles for developers, and such difficulties evolve — for both drug developers and suppliers of raw and starting materials — as a therapy moves through clinical trials and into commercialization.
Our company, Be The Match BioTherapies (BTMB), supports allogeneic-therapy supply chains. Our work starts with identifying and recruiting healthy donors whose cells exhibit the specific attributes that a therapy requires. After that, we collect starting material and transport it for manufacturing, all in compliance with domestic and international regulations.
In our experience, establishing a long-term, strategic partnership between a supplier and developer is critical for success. Our long-term collaboration with Atara Biotherapeutics highlights how a strategic partnership enables a supplier and developer to grow together, refine processes, and negotiate bumps in the road that can occur during the cell-therapy development journey.
Building a Reliable Allogeneic Cell Therapy Supply Chain
Since 2016, our company has provided cellular starting material from healthy donors with specific human leukocyte antigen (HLA) haplotypes for use in Atara’s allogeneic immunotherapy platform. Harvested peripheral blood mononuclear cells (PBMCs) are manufactured into T-cell–based treatments for cancer and autoimmune diseases, then cryopreserved as inventory that can be prepared for delivery to patients in a few days. Atara’s ability to produce investigational off-the-shelf immunotherapies depends on a consistent supply of high-quality healthy-donor cells that are sourced according to regulatory standards.
BTMB is a business unit of the US National Marrow Donor Program (NMDP)/Be The Match (BTM), which for 35 years has coordinated procurement and delivery of blood stem cells for hematopoietic stem cell transplantation (HSCT) and managed a registry of now >7 million genetically diverse potential adult donors from across the United States. Those volunteer donors are also part of a global database of >39 million members. Such work has provided BTMB with a framework for initiating allogeneic cell-therapy supply chains — a framework that has evolved as Atara’s pipeline and the cell-therapy industry have matured.
Leveraging Bioinformatics: Although some drug companies are trying to develop allogeneic cell therapies that are HLA agnostic, many other companies still work with products that require such matching. The latter group needs cutting-edge bioinformatics tools that can obtain insights into HLA genetics.
Bioinformatics methods that our team developed for allogeneic HSCT — which requires HLA matching — now are advancing to meet the needs of cell therapy developers. That evolution began as we adapted our methods to address Atara’s requirements. Bioinformatics insights have supported Atara’s clinical programs to ensure that enough inventory is available for partial matching to patient HLA profiles, bolstering efficacy and safety assessments.
Even if a developer approaches our company with no HLA requirements, other desired donor characteristics and regulatory eligibility requirements must be maintained. As the advanced-therapy industry’s needs change, our bioinformatics team continues to develop its screening algorithms to simplify the process of finding therapy-matched source material.
Why Donor Pool Size Should Be a Top Consideration (Even When HLA Is Not): When an allogeneic therapy is in early development stages, donor pool size might not be a primary consideration. However, as an allogeneic cell therapy advances to high-volume clinical applications and ultimately to a commercial environment, donor pool size becomes increasingly important — even if HLA matching is not required.
It takes a surprising number of potential donors in a starting pool to meet supply needs for an allogeneic cell therapy. Our team reviewed frequency data for different genotypes within our donor registry. We wanted to determine the starting pool size needed to identify 10 qualified HLA-matched donors. For example, we analyzed data relating to the 50th most-common HLA genotype for donors who self-reported being Hispanic or Latinx. That ranking might not seem to be all that common, but it must be considered that BTM’s registry lists 462,000 genotypes. We found that we probably would need a donor pool of >600,000 people to secure 10 qualified donors (2). That is before taking other demographics into account and does not factor in donor willingness to participate, which further diminishes the pool.
Adapting Donor Communication to Maximize Participation: Cell-therapy developers and suppliers must consider donor willingness to participate. The hard truth is that not every person who is asked to donate will be willing or able to do so for a given application. That has been true for HSCT and now is true for emerging allogeneic cell therapies.
Ongoing provision of a high volume of healthy, available donors is difficult even with a large, diverse registry. Through our long-term strategic partnership with Atara, we have gained experience that is changing how we communicate with potential donors as we work to maximize their availability.
For example, people joined BTM’s registry understanding that they could be a match for a patient who needs HSCT. Our donor engagement team provides registry members with information and education about BTMB to help potential donors understand the value of emerging cell therapies. The team has developed a webpage with information specifically geared toward answering frequently asked questions and demonstrating that we partner only with companies whose missions support our own — to save lives through cellular therapy. By introducing potential donors to information about our work with allogeneic cell-therapy developers and having dedicated team members answer their questions, our goal is to maximize donor willingness to participate.
How Screening Parameters Can Limit Donor Pool Size: When other screening factors are added on top of willingness to participate, donor pool size become even more critical. In another internal analysis, we investigated how screening parameters can limit the number of available donors.
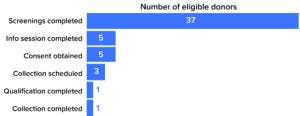
Figure 1: Donor pools for allogeneic-therapy starting material narrow quickly as developers add donor-attribute requirements. Data illustrated here come from a Be The Match BioTherapies analysis of a recent case involving stringent donor requirements ( 3).
Figure 1 shows the number of donors needed after initial screening to perform one collection. The client in this case had stringent additional screening criteria. First, the donor pool was narrowed down from ~7 million potential donors. Those who did not meet demographic requirements (e.g., age, gender, and ethnicity) were filtered out before our team contacted registry members for screening samples. At that point, we evaluated donor samples for specific attributes that were not captured in the registry data — e.g., cell performance in culture or in a particular assay. After information sessions, informed consent procedures, and scheduling, only one subject out of 37 screened donors completed collection (3). This example demonstrates the importance of considering donor pool size and needs for commercial production when selecting a strategic partner for procuring allogeneic starting material.
Broadening Geographic Distribution of Allogeneic Cell Therapies: As shown in Figure 2, regulatory requirements for starting-material collection and processing differ among countries (4–11). Because donor starting material can be used to manufacture hundreds or thousands of doses of an allogeneic therapy, regulatory considerations must be taken into account during clinical trials. A developer does not want to find that collection of starting material for an allogeneic cell bank complied with US Food and Drug Administration (FDA) regulations but not with requirements in the European Union or Australia.
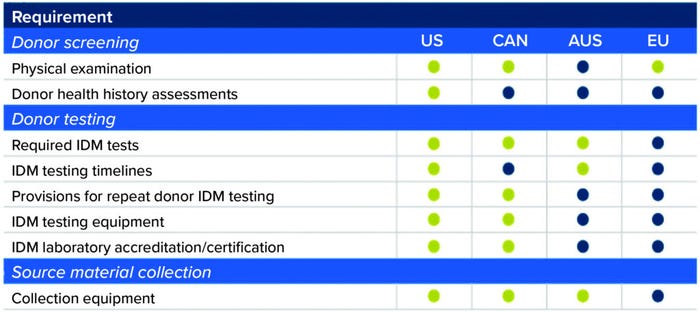
Figure 2: Regulatory requirements for starting material differ across jurisdictions (4–11). Green circles indicate regulatory consistency with US Food and Drug Administration (FDA) guidances. Blue circles represent regulatory differences. (IDM = infectious disease marker; US = United States, CAN = Canada; AUS = Australia; EU = European Union).
BTMB and Atara have collaborated on regulatory compliance from the start. As Atara identified regions for distribution of clinical-trial material, it worked through multiple cycles of process development with BTMB’s regulatory and quality teams to ensure that collection processes met US and international regulations, broadening potential distribution of Atara’s therapies.
In 2021, Atara submitted a marketing authorization application in the European Union for tab-cel (tabelecleucel), making that the first off-the-shelf allogeneic T-cell therapy to be reviewed by a regulatory agency.
Meeting Long-Term Industry Needs
The long-term strategic collaboration between BTMB and Atara demonstrates how a supplier and allogeneic cell-therapy developer can work together to overcome supply chain obstacles as pipeline candidates move from early clinical trials toward commercialization. Lessons that our company has learned from our partnership with Atara and other allogeneic cell therapy developers have led to substantial changes in how we work so that we can meet the growing needs of the allogeneic cell-therapy industry. For example, we are redesigning our operating model to increase our agility and responsiveness, and we are hiring additional staff in key roles to increase our expertise.
As we move into a future in which cell therapy applications will account for an anticipated one-third of our business, the foundation we have built with Atara will help us expand and meet industry needs.
References
1 Regenerative Medicine: Disrupting the Status Quo. Alliance for Regenerative Medicine: Washington DC, 2021; http://alliancerm.org/wp-content/uploads/2022/03/ARM_AR2021_FINAL-singles.pdf.
2 Assessment of 4.5 Million US-Based Be The Match Registry Members in 2020. Be The Match BioTherapies: Minneapolis, MN, 2021.
3 Assessment of Be The Match BioTherapies Client Conversion Rates, 2018–2021. Be The Match BioTherapies: Minneapolis, MN, 2022.
4 21 CFR 1271. Human Cells, Tissues, and Cellular and Tissue-Based Products. US Food and Drug Administration: Silver Spring, MD; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271.
5 CAN/CSA-Z900.1-17. Cells, Tissues, and Organs for Transplantation: General Requirements. CSA Group, November 2017.
6 CAN/CSA-Z900.2.5-17. Lymphohematopoietic Cells for Transplantation. CSA Group, November 2017.
7 2004/23/EC. Setting Standards of Quality and Safety for the Donation, Procurement, Testing, Processing, Preservation, Storage, and Distribution of Human Tissues and Cells. Off. J. Eur. Union 102, 2004: 48–58; http://data.europa.eu/eli/dir/2004/23/oj.
8 2006/17/EC. Implementing Directive 2004/23/EC of the European Parliament and of the Council As Regards Certain Technical Requirements for the Donation, Procurement, and Testing of Human Tissues and Cells. Off. J. Eur. Union 38, 9 February 2006: 78–90; https://eur-lex.europa.eu/eli/dir/2006/17/oj.
9 2002/98/EC. Setting Standards of Quality and Safety for the Collection, Testing, Processing, Storage, and Distribution of Human Blood and Blood Components and Amending Directive 2001/83/EC. Off. J. Eur. Union 33, 8 February 2003: 30–40; http://data.europa.eu/eli/dir/2002/98/oj.
10 2004/33/EC. Implementing Directive 2002/98/EC of the European Parliament and of the Council As Regards Certain Technical Requirements for Blood and Blood Components. Off. J. Eur. Union 91, 30 March 2004: 25–39; http://data.europa.eu/eli/dir/2004/33/oj.
11 TGO 108. Standard for Human Cell and Tissue Products: Donor Screening Requirements. Therapeutic Goods Administration: Woden, ACT, Australia,
24 November 2021; https://www.tga.gov.au/node/941536.
Chris McClain is senior vice president of sales and business development, and Gregg Bodnar is director of client engagement and account management at Be The Match BioTherapies, 500 North 5th Street, Minneapolis, MN 55401; [email protected]; [email protected]; https://bethematchbiotherapies.com.
You May Also Like





