Expanding Considerations in Cleaning Validation: Risks Posed By Indirect Product-Contact Surfaces on Pharmaceutical EquipmentExpanding Considerations in Cleaning Validation: Risks Posed By Indirect Product-Contact Surfaces on Pharmaceutical Equipment
October 20, 2022
Cleaning validation receives a great deal of attention within the biopharmaceutical industry, not least because of the risks of product adulteration and hence patient harm from improperly cleaned surfaces (notwithstanding additional concerns such as operator protection). Traditionally, cleaning validation efforts focus on direct product-contact surfaces. However, the hazard and resultant risk posed from indirect product-contact surfaces should not be underestimated.
Consideration of the risks presented by indirect surfaces should figure into every contamination-control strategy. Understanding of these risks cannot be generalized easily because they depend on equipment design, can be influenced by operational modes (such as a vibrational waves), and even change as equipment ages. Industry surveys suggest that indirect product-contact surfaces are an underexamined area within cleaning validation (1).
Principles
To determine and demonstrate the degree of confidence that a given residue is removed cleaning validation requires gathering and documenting evidence (2). As with most things in the pharmaceutical industry, this requires risk-based assessment (3).
The range of different cleaning technologies includes clean-in-place (CIP), agitation, ultrasonics, and manual processes. As listed in the “Stepwise” box, the common steps of an effective cleaning validation process apply to them all (4–6).
To achieve the necessary understanding and analysis, assessment of cross-contamination risks is required for both the equipment and its surrounding environment (thus accounting for facility and equipment design and use, personnel and material flows, cleaning processes, dirty and clean hold times, storage of cleaned equipment, and the possibility for recontamination). The results of this risk assessment must be reported a formal document that will be updated regularly. An additional consideration comes with risk presented by indirect product-contact sites.
Herein I consider contamination considered coming from two sources (7):
• extrinsic-source active residues such as cleaning-agent residues, material degradants, leachables, and extractables
• intrinsic-source active residues from a product, medium, or buffer that comes into contact with equipment or components.
Microbial-transfer considerations are important and have been addressed elsewhere (7).

Figure 1: Simple representation of cleaning validation objectives.
Confidence in Cleaning
In essence, the key concern in risk assessment of cleaning validation is with how much of residue A will end up in product B after a cleaning procedure has been performed (Figure 1). Cleaning can be evaluated in different ways, which fall into a scale of confidence (Figure 2).
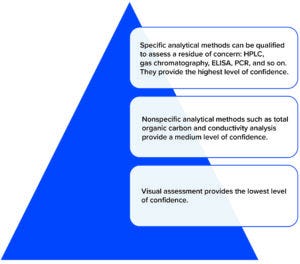
Figure 2: Hierarchy of cleaning residue detection methods.
Visual assessment methods are inherently weak. Human eyesight is limited in its ability to discern “visually clean,” which is complicated by the variables of residue characteristics, foreground–background contrast, wavelength and intensity of the light source used, and personnel experience. Some authors have argued that a visual inspection process can be consistent for many types of residues (8).
In selecting methods for examination, limits set for carryover of product or other residues (including cleaning agents) between equipment uses should be based on toxicological evaluation (9), particularly where residues are known to remain pharmacologically active. Such assessments can be made, for example, using steps listed in the “permitted daily exposure” approach described in the ICH Q3C guideline (10).
The risk of carryover is highest at the point of change-over between product campaigns. Selected limits should be justified in a documented risk assessment. Teams setting acceptance criteria should consider the potential cumulative effect of multiple items of equipment in a process-equipment train. Ideally, known residues should be assessed using specific methods such as high-performance liquid chromatography (HPLC), ion-selective electrodes, flame photometry, derivative UV spectroscopy, enzymatic detection, and titration. If it is infeasible to test for specific product residues, then general methods can be selected such as tests for total organic carbon (TOC) and conductivity.
For purposes of cleaning validation, surfaces can be divided into three categories: direct-contact, indirect-contact, and noncontact surfaces.
Direct Product-Contact Surfaces: Equipment and material surfaces that can be in direct contact with a product stream or any intermediate (e.g., media and buffers) have the greatest potential to end up in a final product. Obvious examples include intermediate hold tanks, formulation mixers, and filling needles.
Sometimes the definition of direct product-contact surfaces is extended to include surfaces that can come into contact with a product either intentionally or unintentionally. Determining what is unintentional is not straightforward, so it necessitates paying closer attention to indirect product-contact surfaces.
Indirect Product-Contact Surfaces: Stopper bowls, forceps, and so on come into direct contact with product-contact surfaces or parts of equipment that are not exposed to product directly but are exposed to process fluids. Such surfaces include some parts of bioreactors, transfer tubing, chromatography columns, vessels, and recirculating segments of CIP systems.
In some other surface areas, material or contaminants can accumulate and then drain, drop, drip, or be drawn into a product. Such surfaces have the potential to transfer residues to the next process run or present a source of microbial transfer. Sometimes this can result from a failure of components or use of equipment under conditions that fall outside the original specifications. Portions of a CIP rinse tank headspace might not come into direct contact with process fluids. However, if a contamination were to develop on those surfaces, nothing prevents that contamination from being transferred to product-contact surfaces during cleaning (11).
Noncontact Surfaces: Floors, ceilings, equipment exteriors, internal hydraulics and refrigerants, and so on never would come into contact with a product or with either indirect or direct product-contact surfaces.
Additional risk factors relate to the type of surface and its finish. Ideal materials are nonleachable, nonadsorptive, and nonadditive. Their surface finish and treatments would be specified to facilitate cleaning — e.g., ensuring that alloys are electropolished and chemically passivated to limit rouge formation (12).
Assessing Indirect-Contact Surfaces and Parts
It is good practice to assess all indirect product-contact surfaces to determine whether they could contaminate product-contact surfaces (13). Some indirect product-contact surfaces might transfer contamination to product-contact surfaces through items or materials. Indirect-contact surfaces can retain product that could be dislodged or transferred into future batches. Hence, airborne particles could end up on such surfaces (e.g., in a lyophilizer). If residues remain and are in close proximity with open product, then a vector (airflow or an operator) could transfer those residues and thus represent a product risk. Surfaces that are in close proximity to open product would seem to present the greatest risk. This depends on materials of construction, design, geometry, and accessibility of equipment.
Some indirect-contact surfaces can be affected by equipment operation, including vibration effects. Examples include the rocking-motion mechanisms of wave-style disposable bioreactors and the stainless-steel housings of single-use stirred-tank vessels. So although single-use technologies reduce the necessity for cleaning validation, the need to ensure effective cleaning cannot be eliminated entirely — and the vibrational effect provides an additional complexity for consideration. Other indirect-contact surfaces are not designed for easy cleaning. It is important to evaluate the level of difficulty in removing product residue from indirect product-contact surfaces. The relative ease or difficulty of cleaning is influenced by surface type, geometry, and design of equipment and components.
In addition, some noncontact parts can come into contact with product in nonideal situations, such as due to faulty equipment operation. Normally noncontact parts of greatest concern are those into which product can migrate: e.g., seals, flanges, mixing shafts, and heating elements.
The degree of risk presented by indirect product-contact surfaces depends on the level of cleanliness that can be obtained (7). This can be assessed through visual inspection and testing of residual levels that may accumulate. Such investigations can be hampered by component accessibility. Visual clues on surfaces, especially where transfer to a direct product-contact site is possible, include
• dry-powder films
• flakes (thick or thin)
• dry, loose granules (large, small, or fine)
• liquids and suspensions
• wet, loose granules (large or small lumps)
• wet smear layers
• compact, surface-bound cakes
• masses resulting from high shear and mixing action
• dried smears resulting from liquid or solvent evaporation
• sticky/greasy masses.
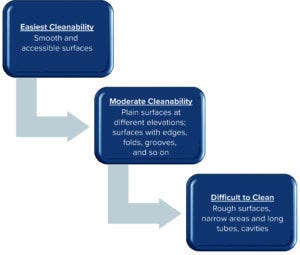
Figure 3: Risk criteria for cleaning validation location selection.
Surface design presents a further risk factor. Figure 3 organizes surface geometries into a hierarchy of cleanability ease.
Risk assessment should be conducted for each indirect product-contact surface. This activity provides a guide for whether indirect product-contact sites need to be included among the sampling locations of direct product-contact sites. Established risk-management tools are useful in preventing selection of sampling sites from being made arbitrarily, as they too often can be. A risk-scoring approach is based on the identification of hazards and assessment of their associated risks: risk level = impact (or severity) × occurrence (or probability). Severity and occurrence can be expressed using a numerical scoring system similar to that in failure modes and effects analysis (FMEA). Table 1 lists core criteria in general terms.

Table 1: Risk criteria for cleaning-validation location selection.
With FMEA, hazards are the adulterants (product residues, contaminants, and so on), and the objective is to identify them and understand potential “failure modes” in relation to indirect product-contact sites that could lead to transference to product-contact sites or directly to a product. In the case of freeze-dryers, a potential failure mode might be presented as residues from a previously manufactured product (a in Figure 1) spilled onto trays and without effective cleaning then potentially transferred to vials of the next product (b in Figure 1) to be lyophilized. In another scenario, residues could accumulate in a crevice and then drip down into the product path of a holding tank. Air currents can dislodge residues from surfaces, and the resulting airborne residues could deposit into a product.
After ranking the severity and occurrence of each surface condition, then a risk priority number (RPN) can be calculated to make risks posed by different indirect product-contact surfaces comparable. This methodology also can be applied to product-contact surfaces (14).
However, detection itself is not risk mitigation. A company will want to know whether its methods can detect residues of concern reliably and with the appropriate limit of quantitation (LoQ). Either the cleaning achieved is acceptable, or it is unacceptable.
In the case of several, similar locations, a validation team can combine and rate indirect product areas where they are exposed to the same level of the contaminant residue and where they are the same types of surfaces or similarly located. A risk assessment would need to be performed for different product types and processes based on equipment use.
In risk assessment, it is good practice to use a multidisciplinary team. Its members should assess indirect product-contact parts in relation to the potential for residue build-up and transfer to product contact sites. Then the team can assess those in increasing order of their difficulty to clean based on accessibility, material type, spatial geometry, and so on.
When it comes to validation, the worst-case residues need to be selected. Regulators expect validation teams to consider solubility, binding potential, toxicity, potency, cleanability, and operational experience, all justified with a risk-based rationale. The task is easier when visible residues are present. It can be aided by an equipment disassembly review, in which equipment is examined after disassembly to identify both product-contact areas and indirect-contact areas that are the most difficult to clean. Just because a residue is not visible, however, doesn’t mean that it won’t present a risk. Several assessments might be required if residue formation is nonuniform. Wherever contamination is visible, its nature on different surfaces will be different (e.g., compacted, dried on, or baked on), and the geometry of the equipment surfaces will determine higher risk areas.
As with direct-contact surfaces, when assessing the shared areas between products A and B (with shared areas that could be different from those between products C and D), indirect-contact sites can present different risks to different processes depending on time, number of additions, and process complexity.
Once the risk-assessment exercise is complete, the team should document its determination of sampling sites for each system along with appropriate justification for each validation exercise. That should be supported by a policy document containing the approach used to determine the most difficult sites to clean in both direct- and indirect-contact areas.
Vibration: An Added Risk
Some items of pharmaceutical equipment are designed to vibrate (producing an oscillation effect or a required force, such as centrifugation). In many cases, however, such vibration can be a concern not just for equipment operability, but also as a mechanism for contamination transfer such as from an indirect- to a direct-contact surface. Vibrodiagnostic studies have demonstrated how particles (especially powders) can be moved across surfaces by vibration oscillations (15). Other items of equipment might slip outside their design parameters and produce unintended vibrations. That can result from a number of conditions, acting alone or in combination, such as imbalances and wear, loose bearings and connections, and misalignments or shaft runout.
Imbalance: A rotating component will cause vibration when unbalanced weight rotates around the machine’s axis, creating a centrifugal force. Imbalance can arise from manufacturing defects (e.g., machining errors) or maintenance issues (e.g., deformed/dirty fan blades and missing balance weights). With increasing machine speed, the effects of imbalance intensify.
Misalignment or Shaft Runout: Radial shaft runout describes radial displacement during the turning of a shaft such as that supporting an impeller. Contributing factors to radial runout include straightness, drive/shaft alignment, and bearing stiffness and wear. Vibration can result when machine shafts are out of line or come from angular misalignment when the axes of a motor and pump are not parallel.
Wear: As components such as ball or roller bearings, drive belts, or gears become worn, they can cause vibration.
Looseness: Vibrations can arise from loose bearings or when a component is attached to its mounts too loosely.
Aging and Modified Equipment
Some biomanufacturing activities can induce abrasion and corrosion. Consideration must be given to any aspect of cleaning for aging equipment. A key principle of hygienic surface design is prevention of macroscopic faults such as crevices, sharp corners, and scratches. Such faults can make cleaning processes less reliable to the degree that repairs are required to maintain a validated state. Good manufacturing, engineering, and maintenance practices should minimize or eliminate wear in part through conducting regular inspections of equipment. Care also must be taken with engineering modifications that could alter equipment functionality that might make indirect-contact surfaces more prone to shed contamination or cause nonproduct-contact parts to come into contact with product inadvertently.
Concerns over legacy equipment should be captured and fed back into a company’s quality by design (QbD) process to ensure that newly specified equipment does not possess the same design flaws. Information and knowledge gained from pharmaceutical development studies and manufacturing experience should provide scientific understanding continuously to support the establishment and maintenance of design space, specifications, and manufacturing controls.
Location Selection and Cleaning Requirements
Risk assessment of indirect product-contact parts informs a validation team about the selection of locations for monitoring as well as requirements for cleaning those areas. Wherever risks are high, the same level of cleaning and sampling should apply to indirect-contact parts as would apply to direct product-contact parts (16). A complication can arise when an equipment design makes indirect-contact parts difficult to surface-sample. In such scenarios, greater importance should be placed on cleaning and ensuring that both detergent contact and rinsing will be adequate in the indirect surfaces of concern.
It is good practice to take digital pictures of such surfaces, areas, and sites with all the parts disassembled. In a photographic library, arrows and other notations can be used to point to specific locations of concern on each picture.
Once all locations have been selected, a cleaning validation assessment can run accounting for worst-case conditions. Test design will need to include consideration of active product solubility and toxicity, the smallest batch size that can be manufactured using the equipment, the maximum daily dose of the associated product; the number of dosages that can be made from a subsequent batch (if contaminated), the highest daily dose, the total area with which a product comes into contact, and the total amount of residual contaminant possible. All locations should be reassessed following changes to the cleaning method and/or process, the introduction of new equipment, and the addition of new products to a manufacturing facility (17).
Assess the Risks to Manage Them
A number of risk factors must be considered in relation to indirect product-contact surfaces for cleaning validation. I encourage companies to increase consideration of such surfaces when outlining cleaning validation processes, especially in regard to sample selection and cleaning-verification assessments. Once areas of concern have been identified, appropriate limits need to be set to identify risks of cross-contamination from indirect surfaces, especially wherever equipment is shared across different process streams. This requires knowledge of which indirect surfaces pose the greatest likelihood of contamination transfer together with an understanding about the performance limitations of different cleaning procedures. Working through this risk-assessment exercise will help companies improve their compliance with good manufacturing practices (GMPs) and ultimately reduce the incidence of product contamination.
References
1 El Azab W, Belkhamassi L. Scientific Justification for Monitoring Indirect Product Contact Surfaces. Cleanroom Technol. 21 October 2019; https://www.cleanroomtechnology.com/news/article_page/Scientific_justification_for_monitoring_indirect_product_contact_surfaces/159191.
2 Tanyous JN. Cleaning Validation: Complete Guide for Health-Based Approach in Chemical Cross Contamination Risk Assessment. PDA J. Pharmaceut. Sci. Technol. 73(2) 2018: 204–210; https://doi.org/10.5731/pdajpst.2018.008946.
3 Agalloco J. “Points to Consider” in the Validation of Equipment Cleaning Procedures. PDA J. Pharmaceut. Sci. Technol. 46(5) 1992: 163–168.
4 LeBlanc DA. Establishing Scientifically Justified Acceptance Criteria for Cleaning Validation of Finished Drug Products. Pharmaceut. Technol. 22(10) 1998: 136–148.
5 Pluta P. Cleaning Validation Failure: Unknown HPLC Peaks Validation Case Study #4. J. Valid. Technol. Autumn 2010: 65–69.
6 Sandle T, Satyada R. Determination of the Cleaning Efficiency for Glassware in the Pharmaceutical Microbiology Laboratory. Eur. J. Parent. Pharmaceut. Sci. 21(1) 2016: 16–22; https://www.academia.edu/24747905/Determination_of_the_cleaning_efficiency_for_glassware_in_the_pharmaceutical_microbiology_laboratory.
7 Sandle T. Microbiological Aspects of Cleaning Validation. J. GxP Compliance 21(5) 2017: 1–12.
8 Melchore JA. Sound Practices for Consistent Human Visual Inspection. AAPS PharmSciTech 12(1) 2011: 215–221; https://doi.org/10.1208/s12249-010-9577-7.
9 Kanegsberg B, Chawla M. How Clean Is Clean Enough? J. Advanc. Applic. Contam. Control 3(9) 2000: 9–12.
10 ICH Q3(R8). Impurities: Residual Solvents. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2021: https://database.ich.org/sites/default/files/ICH_Q3C-R8_Guideline_Step4_2021_0422_1.pdf.
11 Dryness A. Process & Product Contact Surfaces in Bioprocessing. Pharmaceut. Eng. January–February 2017; https://ispe.org/pharmaceutical-engineering/january-february-2017/process-product-contact-surfaces-bioprocessing.
12 Rupesh S. Pressure Vessels for Biomanufacturing. BioProcess Int. 20(6) 2022: 38–42; https://bioprocessintl.com/upstream-processing/bioreactors/pressure-vessels-for-biomanufacturing-basic-considerations-for-cleaning-and-process-compatibility.
13 Rivera E, Lopolito P. Evaluating Surface Cleanliness Using a Risk-Based Approach. Pharmaceut. Technol. 41(12) 2017: 28–37; https://www.pharmtech.com/view/evaluating-surface-cleanliness-using-risk-based-approach-0.
14 Gorsky I. How Clean Is Clean in Drug Manufacturing, Part 2. Am. Pharmaceut. Rev. 18(1) 2015: https://www.americanpharmaceuticalreview.com/Featured-Articles/172929-How-Clean-is-Clean-in-Drug-Manufacturing-Part-2.
15 Lazovic T, Mitrovic R, Marinkovic A. Influence of Abrasive Wear on the Ball Bearing Service Life. Second European Conference on Tribology (ECOTRIB) Conference Proceedings. University of Pisa Engineering Faculty: Pisa, Italy, 2009; 387–392; https://www.academia.edu/26246312/Influence_of_Abrasive_Wear_on_the_Ball_Bearing_Service_Life.
16 Jenkins KM, Vanderweilen AJ. Cleaning Validation: An Overall Perspective. Pharmaceut. Technol. 18(4) 1994: 60–73.
17 Mahajan A. Review on Selection of Worst-Case Product for Cleaning Validation. J. Pharmacy Pharmaceut. Sci. 10(93) 2021: 1–6; https://www.rroij.com/peer-reviewed/review-on-selection-of-worst-case-product-for-cleaning-validation-89287.html.
Tim Sandle is head of microbiology at Bio Products Laboratory in Elstree, UK, and a visiting tutor at both University College London and the University of Manchester; [email protected].
You May Also Like





