Biosafety Testing of Biologicals for Mycoplasma ContaminationBiosafety Testing of Biologicals for Mycoplasma Contamination

Mycoplasma contamination of cell culture (both of primary and continuous eukaryotic cell lines) is common and represents a significant issue of importance in the basic research, development, and production of biologicals. Contamination can alter virtually every physical and chemical property of cells (depending on the contaminating species and the cell type), potentially leading to unreliable results and perhaps unsafe biologicals, biopharmaceutical drugs, or viral vaccines. In fact, contamination may be present with no obvious change in the host culture, even when the concentration of Mycoplasma exceeds that of the host cells by 10- to 100-fold. Thus, testing for Mycoplasma contamination during development and manufacturing is a requirement by the worldwide regulatory authorities in the United States, Europe, and Japan. Key regulations are defined under European Pharmacopoeia (EP) section 2.6.7 (1), Japanese Pharmacopoeia (JP) section 9 (2), FDAPoints to Consider (PTC) documents in 1993 (3) and 1997 (4); 21 CFR 610.30 (5), and the recently announced United States Pharmacopoeia (USP) monograph that will become effective in October 2010 (6)
Licensed biological products produced via cell substrates (e.g., viral vaccines, monoclonal antibodies, and similar products) must be tested to ensure the absence of Mycoplasma contamination. In addition, live viral vaccines produced from in vitro living cell cultures before clarification or filtration (and inactivated viral vaccines produced from living cell cultures before inactivation) must also be tested to ensure the absence of Mycoplasma. Furthermore, tests for the presence of mycoplasma contamination in master cell banks (MCB) and working cell banks (WCB) originating from metazoan cells should also be conducted as part of purity testing.
As there is no single test capable of detecting all strains of Mycoplasma, a combination of assay methods are used for the detection of such contamination. These methods include direct cultivation in broth and on agar media, inoculation onto indicator cell line(s) with subsequent Hoechst staining, as well as nucleic acid detection–based techniques.
Regulatory Differences
Although a level of harmonization exists, some differences exist between the US, European, and Japanese regulatory guidelines.
Qualification Testing: The pharmacopoeias (EP, USP, and JP) require testing for inhibitory conditions only once for a given product. This qualification (“mycoplasmastasis”) testing entails the recovery of spiked control organisms in the presence of test product. The acceptance criteria for the test for nutritive properties of media and qualification testing in the EP and USP methods are aligned for broth culture (1, 6). The growth of Mycoplasma in the presence of test product must be within one subculture of that in absence of test product. For the direct agar portion of the test, however, the quantitative evaluation in the USP is more stringent than it is in the EP. The USP states that the test is compliant if the recovery of the spike organisms is within 0.5 log in the presence of the test material as compared with that in absence of test material. By contrast, the EP defines that if the plates inoculated with spiked test product have less than one-fifth the number of colonies of those inoculated without the test product, then inhibitory substances must be present.
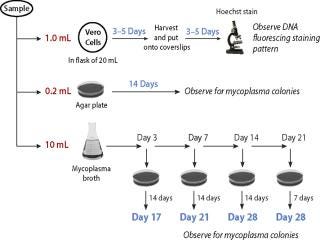
Figure 1: ()
Culture Method Conditions: There are also subtle differences in the incubation conditions between the methods. The EP defines a temperature range of 35–38 °C for incubation, while the USP, CFR, JP, and PTC stipulate it as 36 ± 1°C. Similarly, the terminology for atmospheres slightly varies between the methods. The CFR, PTC, and JP refer to it as “anaerobic” incubation, while the EP and USP use the term “microaerophilic” (although the definition remains identical across the regulations at 5–10% CO2 in nitrogen). All regulatory documents specify the number and types of mycoplasma positive controls to be included in the assays; for CFR, PTC, USP and JP at least two known Mycoplasma species are required, one being a dextrose fermentor and one an arginine hydrolyzer. The EP requires the use of at least one of six Mycoplasma species listed in the chapter as a positive control.
All methods require subculturing throughout the 28-day period (Figure 1). These range from two subcultures for CFR (day 3 and 14), three subcultures for PTC and JP (days 3, 7, and 14), and four subcultures for the USP and EP — with additional subculture on day 21 incubated for seven days. This fourth subculture gives added sensitivity without increasing the duration of the assay.
Detection by indicator cells: The use of indicator cells to detect noncultivable Mycoplasma is also included in specific regulations. Vero cells are most commonly used, but another cell substrate may be used if equivalence for detection of mycoplasma is demonstrated. In the PTC and JP, the test article is added to the cells and incubated for 3–5 days before direct staining and examination by epifluorescence microscopy. Under both the EP and USP methods, however, the test article is added to indicator cells in a flask and allowed to grow for 3–5 days before passaging onto coverslips for a subsequent 3–5 day growth period. This passaging allows for additional growth of the potential contaminants and enhances their detection in the test product.
The degree of alignment amongst these monographs makes it more practicable to satisfy the regulatory expectations under a single assay system. However, further alignment in the monographs should aid in achieving a more concise assay.
About the Author
Author Details
Alison A. Armstrong is a senior director and Doli Patel is a senior scientist, Development Services, at BioReliance, West of Scotland Science Park, Todd Campus, Glasgow G20 OXA, UK. Marian McKee is a principal scientist, Development Services, at BioReliance, 14920. Broschart Road, Rockville, Maryland, 20850, USA.
REFERENCES
1.) European Pharmacopoeia6th Edition.
2.) Japanese Pharmacopoeia XIV, 9.
3.) 1993.Center for Biologics Evaluation and Research, Food and Drug AdministrationPoints to Consider in Characterization of Cell Lines to Produce Biologicals.
4.) 1997.Center for Biologics Evaluation and Research, Food and Drug AdministrationPoints to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use.
5.) Code of Federal Regulations.
6.) General Chapter 63 2010.Mycoplasma TestsUSP 33–NF 28, US Pharmacopoeial Convention, Rockville:88-91.
You May Also Like





