Implementation of the BPOG Extractables Testing Protocols: Working with Multiple Single-Use ComponentsImplementation of the BPOG Extractables Testing Protocols: Working with Multiple Single-Use Components

PALL LIFE SCIENCES (WWW.PALL.COM)
Single-use technologies offer significant advantages over traditional stainless-steel solutions for biopharmaceutical manufacturing. Reductions in setup times, cleaning and cleaning-validation costs, elimination of cross-contamination risks, and smaller footprints are just some of the benefits they provide. Although adoption of single-use systems (SUS) for commercial manufacturing is expanding, concerns persist that extractable and leachable (E&L) compounds from plastic SUS components potentially can leach into final drug products and compromise efficacy and safety. Those concerns are magnified amid the growing number of SUS suppliers and the complex supply chain for SUS and disposable components. In addition, although all equipment used for manufacturing biologic drug substances and drug products must meet current good manufacturing practice (CGMP) requirements (1) and be free of materials that can affect the safety or efficacy of drug products (2), no specific regulatory guidelines or industry standards pertain to performance of E&L studies for SUS.
To provide SUS suppliers with a common format for conducting extractables studies and reporting results, the BioPhorum Operations Group (BPOG) — an industry trade group that represents leading biopharmaceutical manufacturers and SUS end users — published a standardized extractables testing protocol (3). Sharing this knowledge with the biopharmaceutical manufacturing industry not only facilitates SUS adoption, but is crucial to furthering development of effective and practical standardized protocols for disposable technologies. Here we describe some analytical challenges faced when implementing the BPOG protocol for four SUS components.
Implementation of the BPOG Protocol
The BPOG protocol for extractables testing covers sample preparation, extraction conditions, data recording, and results reporting. Implementation efforts include determining the most appropriate extraction conditions and optimizing existing analytical methods for extractables testing. That also involves selecting suitability and internal standards, semiquantification of organic extractables, and elemental impurity characterization using external laboratories. The specifics of the implementation process are provided below for four different components: both a biocontainer film and port, a sterile connector, and a polyethersulfone (PES) filter.
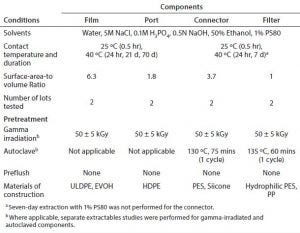
Table 1: Extraction conditions; ULDPE = ultra low density polyethylene; HDPE = high density polyethylene; PES = polyethersulfone; PP = polypropylene
Extraction Conditions: Table 1 summarizes conditions used to characterize the extractable profiles for the biocontainer film and port, sterile connector, and PES filter. Figure 1 presents the extraction setups for the components tested. For each setup, a negative control was generated under the same extraction conditions as for the test article to determine background and system-related signals. The construction of extraction setups to required BPOG specifications is described below.
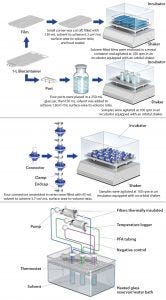
Figure 1: Film extraction setups for the four SU components
Biocontainer Films: Because biocontainers are offered in a number of size, port, and tubing configurations, the film material was characterized independently of port and multisupplier tubing configurations. Specifically, 1-L biocontainers were manufactured on standard equipment without the port or attached tubing and then gamma irradiated at 50 ± 5 kGy. A small corner of each biocontainer (internal surface area = 813 cm2) was cut and filled with solvent (130 mL) to achieve ~6:1 cm2/mL surface-area-to-volume ratio (SAVR). The small cut corner was then heat sealed. Then each biocontainer was placed in a secondary metal container, and the samples were agitated using an orbital shaker inside an incubator at ~100 rpm, which allowed continuous displacement of any small air bubbles trapped during the filling process. Tare and fill weights of the biocontainers were recorded to determine solvent loss during extraction.

Figure 2: Residue accumulation on MS components
Biocontainer Ports: To characterize the injection-molded biocontainer ports, four gamma-irradiated HDPE biocontainer ports with a total fluid contact surface area of 274.3 cm2 were placed in a clean 250-mL glass container (Figure 2) and extracted dynamically with solvent (150 mL) for a total SAVR of 1.8 cm2/mL. Although the three-dimensional geometry of the ports limited the practical SAVR that could be achieved, the ratio was still higher than the surface area of port material per biocontainer extraction volume if the testing had been performed with ports attached, leading to an improved characterization. The enclosed samples were agitated using an orbital shaker inside an incubator at about 100 rpm.

Figure 3: Damage to gas chromatography– mass spectrometry liners
Sterile Connectors: Following sterilization either by gamma irradiation (50 ± 5 kGy) or autoclaving, four pairs of connectors (0.5-in i.d.) with sanitary end fittings were assembled in series and connected (using PTFE gaskets) to each other by securing the junctions using tri clamps (Figure 3). The ends were closed using stainless steel end caps and further secured with tri clamps to ensure no leakage during the extraction study. This test configuration yielded ~50-mL of sample volume per extraction, which was sufficient for all analytics except NVR (nonvolatile residue). In total, more than 500 individual sterile connectors were required for extractables testing. For each extraction setup, the optimized SAVR for the opaque, sterile connector was 3.7 cm2/mL, which greatly exaggerated conditions of use. The samples were agitated using an orbital shaker inside an incubator at ~100 rpm.
Filter Capsules: Following sterilization by gamma irradiation (50 ± 5 kGy) or autoclaving, the filters were connected to PTFE air-driven pumps using perfluoroalkoxy alkane (PFA) tubing and then thermally insulated to help maintain the temperature. The test fluid, which was held in a clean glass reservoir, recirculated through the filters at a flow rate of about 2 L/min. Test fluid temperature was controlled using a thermostat-equipped water bath and continuously monitored immediately downstream of the filter capsule.
Analytical Methods
Before execution of the BPOG protocol, we conducted further optimization of existing in-house analytical methods for extractables testing. These methods include headspace gas chromatography (GC) with mass spectrometry (MS) for volatile organic compounds, direct injection GC/MS for semivolatile organic compounds, and liquid chromatography (LC) with photodiode array (PDA) and/or MS for nonvolatile organic compounds. They are specifically designed to detect a wide range of compounds that may be expected, considering the chemical makeup of the materials of used in our single-use products.
Method Optimization and Initial Screening: The method-development phase consisted of optimizing instrument parameters and sample pretreatment using a standard mixture of typical extractables from our materials of construction. This improved the specificities of the methods and achieved detection limits as close as possible to BPOG’s reporting threshold (0.1 ppm). The optimization process included selection of the most suitable GC and LC columns, mobile phase compositions, system suitability and surrogate standards, and sample pretreatment(s) procedures (such as liquid-liquid extraction, concentration, and dilution); and evaluation of the applicability of internal standards for each method.
For LC/PDA/MS analyses, prescreening using UPLC/PDA/ Q-ToF was conducted with 50% ethanol, high pH, and low pH extraction samples from autoclaved and/or gamma-irradiated components to improve peak identification and quantification. Because Q-ToF provides highly accurate mass data and offers higher sensitivity and resolution than single-quadrupole mass spectrometers, this initial screening process enabled construction of a compound library of potential extractables with retention times, molecular formulae, and mass-to-charge-ratio (m/z) ion information. This compound library has been used extensively during routine testing for identification and targeting of specific compounds that exhibit poor sensitivity in particular matrices (e.g., through mass ion extraction from total ion chromatograms).
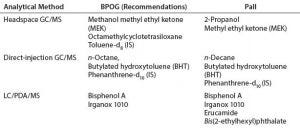
Table 2: BPOG and Pall system suitability standards for organic extractables analyses (IS = internal standard)
Selection of System Suitability Standards: Table 2 summarizes a comparison of BPOG-recommended and Pall Life Sciences system suitability standards for organic extractables analyses. Although they do agree, subtle differences between the two approaches are primarily due to selection criteria specific to Pall, such as our historical data and knowledge of our materials of construction and compounds of toxicological concern.
Specifically, for LC/PDA/MS analyses, erucamide and bis(2-ethylhexyl)phthalate were included in addition to bisphenol A and Irganox 1010 due to frequent end-user inquiries related to the presence of these additives in materials of construction. Erucamide also was found to be highly suitable for assessing system suitability under ES+ mode. For direct-injection GC/MS, n-decane was used instead of the n-octane recommended by BPOG, because n-decane is more frequently observed from our materials of construction.
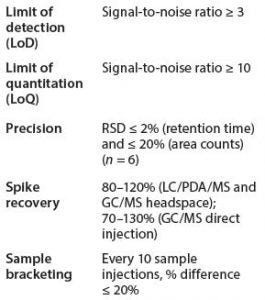
Table 3: Typical system suitability standards for GC/MS and LC/PDA/MS analyses
For headspace CG/MS, we chose 2-propranol rather than methanol (which is recommended by BPOG) because the former is a more common residual solvent from our manufacturing process and materials of construction. Octamethylcyclotetrasiloxane was not included because siloxane detection was optimized through direct-injection GC/MS. We also found that an internal standard was not necessary for headspace GC/MS sample analyses because desired precision and accuracy met consistently using an external standard method. System Suitability Requirements for Organic Extractables Analyses: Typical system suitability requirements for GC/MS and LC/ PDA/MS analyses include limit of detection (LoD), limit of quantitation (LoQ), precision, and accuracy/ recovery (Table 3).
Semiquantification of Organic Extractables and Reporting Thresholds: Many laboratories use system suitability standards for semiquantification of extractables and the response of these standards as the basis for their reporting thresholds. As a result, there is a much greater level of uncertainty in reported quantities because instrument response factors vary greatly from one compound to the next. Hence, reporting thresholds may not reflect the actual detection and quantification limits of each specific compound. This effect may be more pronounced when comparing responses for compounds that are not structurally similar.
Therefore, to obtain more accurate results, authentic compounds (whenever possible) or chemically related compounds (if an authentic reference compound is not available) are used to estimate the concentration of known extractables. The detection and quantification limits of each authentic and chemically similar compound also are evaluated and reported because these data are more meaningful for toxicological risk assessment than reporting thresholds that are based solely on system suitability assessments.
Semiquantification of extractables is performed by comparing the responses of the peaks in a sample with those of an authentic or a chemically similar compound at concentrations close to those of the compounds in the sample (as indicated by their responses). For the four components discussed herein, all unknowns were semiquantified using system suitability standards. Per the BPOG protocol, all data above 0.1 ppm are reported.
ICP/MS Testing for Elemental Impurity Characterization: In-house extracted samples are assayed by a qualified laboratory using an inductively coupled plasma mass spectrometry (ICP-MS) method that complies with industry standard USP <233> and element portfolio matching USP <232> and ICH Q3D. The method is capable of detecting ICH Q3D elements with a target level of 20 ppb. Actual LoD and LoQ values are reported. Before sample analysis, the compendia recommend assessing system suitability requirements for recovery, LoD, LoQ, precision, double-charged ion ratio, and oxide ratio. The recovery is evaluated using spiking studies, with 70−150% as the acceptance criterion, which is aligned with USP <233>. LoD is determined based on three times the standard deviation from blank matrix injections, and the LoQ is based on 10× the standard deviation from the blank matrix injections. Each element is quantified using a four-point calibration curve. All samples are treated with concentrated HNO3/HCl and diluted before analysis, except for 0.1M H3PO4 and water samples, which are injected directly.
Laboratory Challenges
The BPOG protocol requires extraction of single-use components in six solvents. During initial testing, we found that some solvents presented difficulties during sample analysis, primarily in terms of instrument wear and tear and/or analytical interference. The former instance resulted in increased instrument downtime and a need for more frequent maintenance. In most cases, further optimization and instrument troubleshooting were required to limit solvent effects on the instruments and improve instrument sensitivity.
Among the BPOG solvents, 1% polysorbate 80 (PS80) posed the greatest challenge for LC/MS sample analysis. Specifically, PS80 contaminates the mass spectrometer ion source and lens coating (Figure 2), leading to reduced instrument sensitivity after multiple injections. To mitigate that issue, all 1% PS80 samples were diluted 10-fold to minimize contamination. Even with dilution, frequent maintenance was still required. Attempts to remove PS80 completely using solid-phase extraction (SPE) and liquid−liquid extraction (LLE) followed by evaporation and reconstitution were unsuccessful. In addition, PS80 and its related compounds interfered with the sample analyses. For example, PS80 degradation products (PEG-related compounds, aldehydes, fatty acids, and PS80 reaction products with actual extracted substances) significantly overlapped with extractables from our materials of construction and coeluted with other extractables, suppressing their ion signals. Consequently, fewer extractables generally were detected in this solvent. To help mitigate ion-suppression issues, the extractables profile from 50% ethanol/water extraction was used to specifically target potential extractables in 1% PS80 (for example, by mass ion extraction).
Similar difficulties were observed with DI GC/MS analyses of 1% PS80 solutions. Liquid−liquid extraction of 1% PS80 samples with dichloromethane was insufficient. Further washing of the organic phase with water proved more effective in reducing the concentration of PS80 and minimizing impact on the instrument and data interpretation. Because of the adverse effect of PS80, samples were not concentrated before analysis. For all other samples, extracts were initially concentrated 50× before DI GC/MS analysis to improve the detection limits and reduce the risk of missing extractables that could pose toxicological concern. However, because of the instrument wear and tear caused by injecting highly concentrated samples to the GC/MS (Figure 3), 20-fold concentration typically was used. Both 1× and 20× concentrated extracts were injected and analyzed.
Furthermore, injection of 5M NaCl samples to the LC/MS frequently caused severe sensitivity issues based on accumulation of salts on the ion source and probe, which compromised the ionization efficiency. Solvent cut-off (approximately 1 minute) and wash procedures were incorporated into the method to overcome this issue.
The Benefits of a Common Format
BPOG’s standardized extractables testing protocol provides SUS suppliers with a common format for conducting extractables studies and reporting results, which should facilitate adoption of SUS in biopharmaceutical manufacturing. Pall investigated implementation of this protocol for four common SU components.
Extraction setups were established to meet BPOG specifications. Existing analytical methods were optimized to improve specificities and achieve detection limits. In some cases, alternative system suitability standards for the semiquantification of
unknowns were used, primarily due to selection criteria specific to Pall.
The greatest difficulties occurred during sample analysis from wear and tear of instrument parts and interference with analyses by certain solvents required by the BPOG protocol, particularly PS80 and 5M NaCl. Those issues were minimized through further optimization of the relevant methods and through instrument troubleshooting.
Implementation of the BPOG protocol for extractables testing of SU components, although not a simple process, can be achieved using carefully designed extraction setups, optimizing existing analytical methods, and instrument troubleshooting.
In BPI’s January–February issue, we will explore the protocols for evaluating extractables in SU components and offer a comparison of results for autoclaved PES filters obtained using the BPOG protocol and the proposed USP<665> protocol.
References
1 Code of Federal Regulations, Title 21, Food and Drugs (Government Printing Office, Washington, DC), Part 211.65(a).
2 Code of Federal Regulations, Title 21, Food and Drugs (Government Printing Office, Washington, DC), Part 600.11 (b).
3 Ding W, et al. Standardized Extractables Testing Protocol for Single-Use Systems in Biomanufacturing. Pharmaceut. Eng. 34(6) 2014: 1-11; www.biophorum.com/wp-content/uploads/2016/10/17_file.pdf.
Corresponding author Gilbert Tumambac, PhD, is senior principal scientist, SLS regulatory and validation consulting Group, Pall Life Sciences, [email protected]. Benben Song, PhD, is principal scientist, Chunxiu (Tina) Xing is scientist II, Gang Li, PhD, is senior scientist, Wenan Lu, PhD, is scientist II, Lin Liu is scientist II, Chien-Ju (Cherry) Shih, PhD, is scientist II, and James Hathcock, PhD, is senior director, all at Pall Life Sciences.
You May Also Like






