A New Generation of Generic HCP AssaysA New Generation of Generic HCP Assays
August 1, 2013

Host-cell protein (HCP) analysis is a big issue when it comes to early clinical testing, design of downstream processes, and quality control of biopharmaceuticals. The decision to use an off-the-shelf generic HCP assay or spend a considerable amount of money for development of a specific HCP assay is often not an easy one. In theory, generic HCP assays should be suitable for all HCP determinations of a specific cell line — independent of cell line modifications, fermentation conditions, and the design of the purification process. But in most cases, generic HCP assays do not show the desired specificity and sensitivity.
Enhanced Generic Assay to Detect Host Cell Proteins
BioGenes has developed an enhanced generic CHO 360-HCP assay that is demonstrably superior to current generic HCP assays, allowing sponsors and biotech companies to postpone the development of a cost-intensive specific HCP assay until a more informed decision on the success of a biologic in development can be made.
The CHO 360-HCP assay employs anti-HCP antibodies from different species. By immunizing rabbits and goats with HCPs of a nontransfected Chinese hamster ovary (CHO) cell line — combined with a unique approach for antigen and antibody preparation as well as an optimized purification strategy — BioGenes has developed a panel of four different HCP enzyme-linked immunosorbent assays (ELISAs, type A to D) that together build up the enhanced generic CHO 360-HCP assay.
For all four assay types, the lower limit of detection (LOD) is between 0.5 and 1.0 ng/mL, and the lower limit of quantification (LOQ) is 2–3 ng/mL, with a working range of 2–100 ng/mL. A high specificity for the antigen in all four assays was determined by Coomassie staining of the CHO-HCP antigen and 2D Western blotting with the respective antibodies.
Superior HCP Recovery
Designed to cover a broad spectrum of antigens, the CHO 360- HCP assay has been widely tested on the basis of a great number of mock CHO HCP samples (Figure 1). In the case of sample 4 (x), a recovery of >90% was estimated using assay type D. Recovery with assay types A–C was much lower, and recovery with the commercial assay was only 20%. In most cases, the best recovery was estimated with one of our four generic CHO-HCP assays (Type A to D).
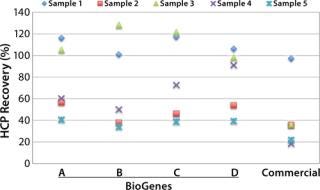
Figure 1: ()
Based on our data and long lasting experience, we conclude that there is no single generic assay suitable for all samples. However, the integration of four different assay types in our CHO 360-HCP assay — together with an upfront feasibility test to identify the most suitable antibodies — does greatly increase the recovery for most samples. It’s time to rethink generic HCP assays. It’s time to choose the antibodies that work best for you!
About the Author
Author Details
Yvonne Haberkorn, PhD, is product manager immunoassays, Koepenicker Str. 325, D-12555 Berlin, Germany; +49-30-65762378; [email protected].
You May Also Like






