A Salt-Tolerant Anion-Exchange Chromatography Sorbent for Flexible Process DevelopmentA Salt-Tolerant Anion-Exchange Chromatography Sorbent for Flexible Process Development
In most downstream purification processes designed for biopharmaceutical drug production, dilution and diafiltration sequences are unavoidable. Such operations are routinely used to adjust a feedstock or chromatographic fraction to the optimal conditions required for best process performances. Nevertheless, those steps are often time, water, and labor consuming without participating directly in final product purification. Because biopharmaceutical production is increasingly driven by cost reduction, a possible means for enhancing process economics is to streamline purification by eliminating these unit operations before or between chromatography steps as much possible.
Anion exchangers — quaternary amine (Q) or diethyl-amino-ethyl (DEAE)sorbents — are commonly used first purification steps in biomanufacturing. However, use of conventional anion exchangers requires low to moderate ionic strength to achieve sufficient capacity (1, 2). Therefore, integrating chromatography sequences with anion-exchanger sorbents often requires dilution or diafiltration to adapt feed conductivity to sorbent limitations (3). Implementation of a “salt-tolerant” anion exchanger would allow direct capture from undiluted feedstock and improve process economics signifcantly (4).
PRODUCT FOCUS: ALLBIOLOGICALS
PROCESS FOCUS: DOWNSTREAM
WHOSHOULDREAD: Analytical manufacturing, process development
KEYWORDS: PURIFICATION, SALT TOLERANCE, DYNAMIC BINDING CAPA#CITY, CHO, HUMAN SERUM ALBUMIN
LEVEL: INTERMEDIATE
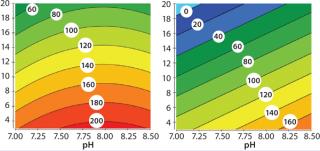
Figure 1: ()
The new HyperCel STAR AX “salt-tolerant” anion exchanger from Pall Life Sciences is designed to provide high dynamic binding capacity (DBC) at moderate to high conductivities over a wide range of pH values and at short residence times. The “salt tolerance” is based on a primary amine ligand, immobilized on a robust, industry-scalable HyperCel matrix.
To assess the performance of this new sorbent, we first evaluated DBC and selectivity using model proteins and compared them with those of a conventional anion exchanger. The sorbent was then challenged in three different “real-case” applications to address the impact of salt tolerance in full purification processes. We evaluated HyperCel STAR AX sorbent in bind–elute mode for capture of α-amylase from Chinese hamster ovary (CHO) cell culture supernatant and human serum albumin (HSA) in cryo-poor plasma. Evaluations in negative mode tested the elimination of CHO contaminant proteins to purify recombinant human interleukin 7 (rhIL7).
Materials and Methods
Chemicals and Equipment: Sigma Aldrich provided analytical-grade reagents and α-amylase. Millipore provided bovine serum albumin (BSA). Human plasma and CHO cell culture supernatant (CCS) for α-amylase application was produced within Pallfacilities. CHO CCS for the rhIL7 application was kindly provided by Cytheris (Issy-les-Moulineaux, France). Pall provided S HyperCel and HyperCel STAR AX sorbents, each packed according to manufacturers’ instructions into glass columns from Kronlab. We used Äktaexplorer 100 and Äkta avant 25 systems from GE Healthcare for all chromatographic runs.
Methods: We used several quantification assays to estimate recovery and purity of target proteins (sidebox “Analytical Assays”). Recovery and purity of α-amylase and HSA were calculated as shown in the Equations box. Runs used 1-mL columns (0.5 × 5.0 cm) at 2 min residence time except where otherwise stated (Table 1).
Equations: Calculation of recovery and purity for α-amylase and HSA
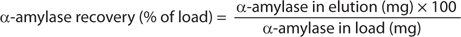
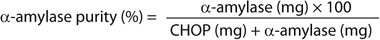
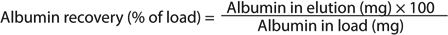
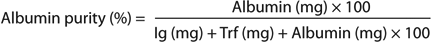
To optimize conditions, we used high-throughput screening on 96-well microplates as described by Toueille et al. using the range of conditions listed in Table 2 (5). We planned and analyzed a design of experiments (DoE) study using Minitab statistical software (Minitab Inc.). For our process economics analysis, we used BioSolve cost of goods (CoG) analysis software (Biopharm Services) and performed real-case applications developments as listed in Table 3.
Analytical Assays Used in Real Case Studies
Total Proteins
Bradford assay kit and bicinchoninic acid (BCA) assay kit (Pierce Thermo Scientific)
Human Serum Albumin
Bromocresol green colorimetric assay (Fisher Diagnostics)
Transferrin, CHO Host Cell Protein (CHOP)
ELISA assay kit (Cygnus Technologies)
Immunoglobulins
Protein A HPLC column (Applied Technologies)
α-amylase
Ceralpha colorimetric assay (Megazyme)
Recombinant human interleukin 7 (rhIL7)
ELISA assay kit (Cell Sciences)
SDS-PAGE
NuPAGE 4–12% Bis-Tris gels and Coomassie SimplyBlue (Invitrogen)
Results and Discussion
Salt Tolerance (Influence of pH and Conductivity on DBC): We evaluated the effects of a wide range of pH (7.0–8.5) and conductivity (3–20 mS/cm) on the DBC for BSA of HyperCel STAR AX sorbent and compared results with those of a standard Q anion-exchanger. Figure 2 shows that the contour plots evidenced different profiles directly linked to the chemistry used to fun
ctionalize each sorbent. Standard Q anion exchangers providehigh DBC at low to moderate conductivity (6). Logically, rigid Q agarose sorbent reaches high capacity (>100 mg/mL) at moderate conductivity but shows no salt tolerance as DBC drops when conductivity increases (40–60 mg/mL at 15 mS/cm). For the HyperCel STAR AX sorbent, the primary amine chemistry ligand we used allows both high DBC and high conductivity. As with other modern anion exchangers, the material delivers very high DBC (≥180 mg/mL) at low conductivity (≤5 mS/cm). It keeps capacity >100 mg/mL at conductivities ≤15 mS/cm.
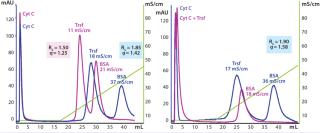
Figure 2: ()
Even under the least favorable conditions for BSA capture, DBC is ~60 mg/mL, and performance of the HyperCel STAR AX sorbent is maintained at residence times as low as 1 min (not shown). Therefore, it displays capacity equivalent to advanced anion exchangers but significantly extends the conductivity operation range. So integration of HyperCel STAR AX sorbent in purification processes offers the possibility of directly capturing protein from undiluted feedstock, thereby eliminating the need for dilution or diafiltration.
Selectivity (Separation of a Model Protein Mixture with a Linear Salt Gradient): Selectivity is an important parameter in the choice of an ion exchanger for a specific process and should be evaluated case by case. For example, we compared HyperCel STAR AX selectivity with that of a rigid Q agarose sorbent by using a mixture of three model proteins. The proteins separated through a linear salt gradient after loading at conductivities of 2 or 10 mS/cm. Figure 3 shows that cytochrome c was not retained on the anion-exchange sorbents because it is positively charged at pH 8.0. The other two proteins were bound and separated from HyperCel STAR AX sorbent with good resolution (Rs) and selectivity (α) performance maintained at different conductivities. Rigid Q agarose sorbent showed a similar separation pattern at low load conductivity, although resolution was lower. That pattern was not maintained when load conductivity increased. For this case, transferrin did not bind, thus leading to a modification of the separation capabilities on rigid Q agarose depending on loading conductivity. Note that elution of transferrin and BSA from HyperCel STAR AX sorbent needs higher salt concentration because of its salt-tolerant properties. However, all bound proteins are completely eluted in the 0–1 M NaCl gradient as with other standard ion exchangers (not shown).
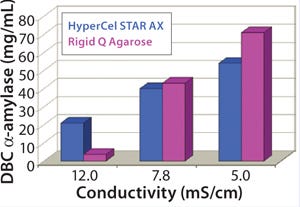
Figure 3:
Selectivity data at different conductivities highlight the robustness of the separation performance of HyperCel STAR AX sorbent independently of conductivity. That provides flexibility in process design and the possibility ofaccommodating feed variations without modifying a separation profile.
Capture of α-amylase from CHO CCS: We evaluated the performance of HyperCel STAR AX sorbent for protein capture from a CHO CCS using α-amylase as a model. To address the effect of feedstock dilution, we evaluated DBC for HyperCel STAR AX sorbent and rigid Q agarose with CCS containing 0.5 mg/mL α-amylase and adjusted by dilution at different conductivities. HyperCel STAR AX sorbent provided the highest capacity at 20 mg/mL with crude feedstock (Figure 3), thereby confirming its “salt tolerance.” The highest capacity for both sorbents was obtained with fourfold dilution (5 mS/cm), but this dilution would be too high for favorable process scale-up economics. Therefore, capture using only crude and twofold diluted feedstock was further investigated.
Figure 4 shows results of standard elution optimization using a NaCl gradient up to 1 M. We observed two peaks for both sorbents during salt elution. The α-amylase activity was detected only in the first peak, whereas the second probably corresponds to nucleic acids (high ratio of A260 nm to A280 nm). For HyperCel STAR AX sorbent, however, the second peak was well separated from the α-amylase peak because it eluted only during the high-salt strip. Therefore, thanks to its strong affinity for nucleic acids, HyperCel STAR AX sorbent provides efficient separation of such contaminants.

Figure 4: ()
On the basis of the elution profiles, we chose elution conductivities for α-amylase purification runs as 48 mS/cm on HyperCel STAR AX sorbent and 34 mS/cm on rigid Q agarose sorbent. We then screened different wash conditions to improve α-amylase purity using the above elution conditions for both sorbents (not shown). Data indicated that a wash step combining low pH and low conductivity (pH 4.5, 2 mS/cm) improved CHOP removal for both sorbents while maintaining α-amylase activity and recovery yield (Table 4).
Overall, our study demonstrated that the HyperCel STAR AX “salt-tolerant” anion-exchange sorbent can efficiently capture and purify biologically active enzymes from both crude and diluted CHO feedstock with equivalent productivity. Thus it can bring process flexibility and robustness to purification processes (Table 5). By contrast, using undiluted feedstock on conventional rigid agarose can decrease productivity by about four times (not shown).
Process Economics for Bind–Elute Mode: To address the effects of salt tolerance of HyperCel STAR AX sorbent on process economics, we developed a two-step process for HSA purification from plasma using anion exchange as capture. Performance of this sorbent was evaluated in parallel with that of a DEAE agarose sorbent, a commonly used type of sorbent in plasma fractionation.
We determined the effect of feed conductivity on sorbent capacity by evaluating DBC of both sorbents using plasma at different dilutions (Figure 5). DBC of HyperCel STAR AX sorbent was maintained around 30 mg/mL with load at conductivities ranging from 4 to 11 mS/cm (dilution one- to threefold). By contrast, an increase of load conduc
tivity significantly decreased DBC of the rigid DEAE agarose sorbent.
Figure 5:
Those data confirm the salt-tolerant behavior of HyperCel STAR AX sorbent and its ability to directly capture HSA with high capacity from nondiluted plasma. Compared with the standard sorbent, the DBC of HyperCel STAR AX sorbent was more than twofold higher and maintained in a broad range of conductivities. That brings greater process robustness and flexibility, by accommodating potential variations in the ionic strengths of feedstocks.
We optimized wash and elution conditions using DoE on 96-well filter plates (Table 2). We determined optimal wash and elution conditions for purity and yield by using response surface modelinganalysis. Contour plots of HyperCel STAR AX sorbent data revealed that HSA purity was significantly affected by wash conditions (Figure 6LEFT). Increase in wash conductivity positively affected HSA purity, with an optimal zone for wash conductivity >15 mS/cm. The optimal elution conditions zone was pH 3.5–4.2 at conductivities of 2–27 mS/cm (Figure 6RIGHT). We chose optimal combinations of wash and elution conditions providing estimated yield and purity ≥90% according to model predictions (Table 6).
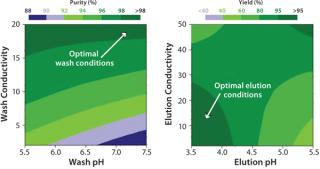
Figure 6: ()
We applied optimized conditions to column runs yielding highly pure HSA and satisfying yield for both sorbents (Table 6). The possibility of eluting from HyperCel STAR AX sorbent using only a pH drop at low conductivity (Figure 7) provides the opportunity to directly load elution from this sorbent on a cation exchange sorbent (orthogonal step). However, the same elution conditions tested on rigid DEAE agarose resulted in good purity but only partial elution (73% yield) (not shown).
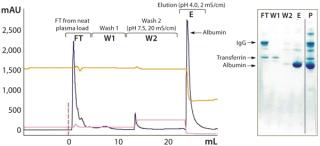
Figure 7: ()
We used HSA purified on HyperCel STAR AX and rigid DEAE agarose sorbents as loading feed to optimize the second purification step of HSA on S HyperCel cation exchanger. Optimization on 96-well plates (data not shown) allowed determination of the best elution conditions at pH 7.0, 15 mS/cm, providing HSA with 95% recovery and >99% purity.
With process economic analysis, we compared the two two-step purification processes for HSA using either HyperCel STAR AX or rigid DEAE agarose sorbents as a first step and S HyperCel as a second step (Figure 8). The pH-driven elution from the capture column provided the most streamlined purification scheme, which appeared promising for plasma fractionation. We compared the “salt-tolerant” and the standard anion-exchanger sorbents using those conditions.
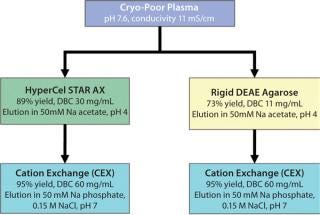
Figure 8: ()
A cost comparison showed that a process using rigid DEAE agarose as its capture step can increase costs by 67% compared with processes using HyperCel STAR AX sorbent (Figure 9). The lower DBC obtained at capture with undiluted plasma necessitates more runs to process the same amount of HSA. That results in a higher buffer consumption, water use, and material/tankage costs. In addition, lower throughput in kg/year (–22%) was achieved as a result of the poorer elution yield from rigid DEAE agarose with low salt buffer.
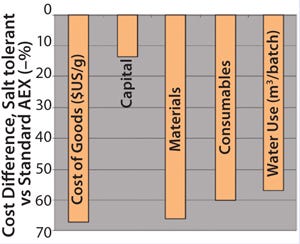
Figure 9:
We also simulated an alternative scenario for a process using optimal loading and elution conditions for capture on rigid DEAE agarose. This case integrated a threefold plasma dilution to maximize HSA capture and applied optimal elution conditions of pH 4.0 and 25 mS/cm to increase yield. Despite better performances for the conventional anion-exchanger, the global cost of this process was still 14% higher than for a process using HyperCel STAR AX sorbent. That is a result of preliminary plasma dilution, followed by a second dilution ofthe first-step elution required for the second purification step on the cation-exchanger (data not shown). This second case study highlights again the performances of HyperCel STAR AX sorbent for purification of proteins in high-conductivity environments and illustrates the positive effects of salt tolerance on process economics.
Process Economics for Flow-Through Mode: Our study included early contaminant removal for the purification of a recombinant human Interleukin 7 expressed in CHO cells. Anion-exchange sorbents are often operated in negative (or flow-through) mode to capture contaminants, which leaves a target protein in the flow-through. We evaluated performance of HyperCel STAR AX sorbent for capture of contaminants from a CHO CCS during a recombinant human Interleukin 7 (rhIL7) purification process and performed a process economics analysis.
We determined the DBC for contaminant proteins on HyperCel STAR AX sorbent compared with that of rigid Q agarose sorbent at different conductivities using crude and twofold-diluted CCS. As Figure 10 shows, the highest DBC was obtained with crude CCS for HyperCel STAR AX sorbe
nt and with twofold diluted CCS for rigid Q agarose. As expected, the standard anion exchanger requires lower conductivity to capture the largest number of contaminants. For HyperCel STAR AX sorbent, the salt tolerance allows efficient binding of contaminants at high conductivity. We obtained high recovery yields in the flowthrough for rhIL-7 (>90%) in all conditions tested for both sorbents.
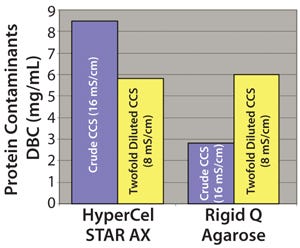
Figure 10:
Results showed that lower conductivity decreases contaminant DBC for the HyperCel STAR AX sorbent. The lower protein concentration in twofold-diluted CCS could negatively affect the adsorption equilibrium of contaminants, thus decreasing total protein capacity. Contaminant DBC of the HyperCel STAR AX sorbent is equivalent to that of rigid Q agarose using twofold-diluted CCS.
We used two different processes for process economics analysis that included either crude CCS (16 mS/cm) or twofold-diluted CCS (8 mS/cm) (Figure 11). When compared with rigid Q agarose, HyperCel STAR AX sorbent always provided significant cost reduction and water savings, regardless of the chosen scenario (Figure 12). The largest decrease of CoG (23%) was shown through comparing the use of both sorbents loading crude CCS (scenario 1). CoG reduction came mostly from potential savings on capital equipment (25%), because a lower capacity of contaminants on rigid Q agarose would require larger column diameter, larger pumping system, and larger vessels for buffer preparation. The savings from using HyperCel STAR AX sorbent were still significant even when loading twofold-diluted CCS (second scenario).
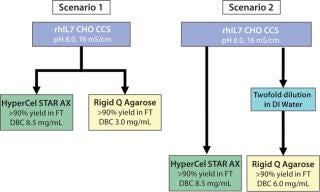
Figure 11: ()
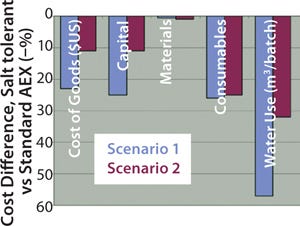
Figure 12:
We scaled up using a 100-mL (2.5 cm × 22 cm) column of HyperCel STAR AX sorbent and positioning it at the first step of a complete purification process for rhIL7 from crude CCS. The trial confirmed the efficiency of CHO contaminant protein removal (not shown).
Our studies illustrate that HyperCel STAR AX sorbent provides strong economical benefits compared with a standard ion-exchange sorbent when used in negative mode.
Next-Generation Tool
The new HyperCel STAR AX “salt-tolerant” anion-exchange sorbent facilitates the protein capture (target or contaminants) from moderate- to high-conductivity feedstocks, limiting dilution or ultrafiltration/diafiltration (UF/DF) requirements. We demonstrated the sorbent’s ability to maintain robust DBC over a large range of conductivity and pH values using model proteins as well as different types of “real” feedstocks (plasma, CHO CCS) with different target proteins. The new sorbent provided a significant advantage for streamlined process development compared with conventional anion-exchange sorbents. It allows for elimination of initial dilution or diafiltration steps usually required to accommodate feedstock for operation on standard anion exchangers. That is possible while keeping purification and recovery performance as well as optimization of operational conditions in line with those of the existing range of conventional anion exchangers. In real-case applications, “salt tolerance” led to significant savings compared with conventional sorbents (decreased CoG for operation of the sorbent in bind–elute mode and in flow-through mode).
The HyperCel STAR AX sorbent appears to be a next-generation chromatography tool for capture steps as well as contaminant removal in various purification sequences. Altogether, the data herein illustrate its functional and economical performance and demonstrate its benefits toward allowing development of more streamlined and cost-effective processes.
About the Author
Author Details
Jerome Champagne, PhD, is senior R&D scientist, chromatography applications at Pall Life Sciences (France). Aleksandar Cvetkovic, PhD, is principal R&D engineer, chromatography applications at Pall Life Sciences (USA). Guillaume Balluet is associate R&D scientist, chromatography applications; Sylvio Bengio, PhD, is scientific communications manager, global chromatography; and Magali Toueille, PhD, is principal R&D scientist, chromatography applications, all at Pall Life Sciences (France). Corresponding author, René Gantier, PhD, is senior R&D manager, Biopharm Applications at Pall Life Sciences, 50 Bearfoot Road, Northborough, MA, 01532; 1-508-351-0193; [email protected] following are registered trademarks: HyperCel (Pall Corporation); Minitab (Minitab Inc.), ÄKTAexplorer and ÄKTA avant (GE Healthcare), and NuPage (Invitrogen).
REFERENCES
1.) Bengio, S. 2010. Improving IEX Throughput and Performance with Differentiated Chromatography Sorbents. BioProcess Int. 8:64-74.
2.) Harinarayan, C. 2006. An Exclusive Mechanism in Ion-Exchange Chromatography. Biotechnol. Bioeng. 95:775-787.
3.) Arunakumari, A, and J Wang. 2009.Purification of Human Monoclonal Antibodies: Nonprotein A Strategies in Process Scale Purification of AntibodiesProcess Scale Purification of Antibodies, Gottschalk U. Wiley Online.
4.) Han, C. 2010. Process Development’s Impact on Cost of Goods Manufactured (COGM). BioProcess Int. 8:48-55.
5.) Toueille, M. 2011. Designing New Monoclonal Antibody Purification Processes Using Mixed-Mode Chromatography Sorbents. J. Chromatogr. B 879:836-843.
6.) Staby, A, I Jensen, and I Mollerup. 2000. Comparison of Chromatographic Ion-Exchange Resins: I. Strong Anion-Exchange Resins. J. Chromatogr. A 897:99-111.
You May Also Like






