Screening Yeastolate Raw Material Used in Insect Cell Culture MediaScreening Yeastolate Raw Material Used in Insect Cell Culture Media
Insect cell culture is widely used as an industrial platform in recombinant protein production for research and diagnostics (1, 3,4,5). Many commercial yeastolate products are intended as nutritional supplements for not only insect cell culture, but also for mammalian cell culture and bacterial fermentation applications (6,7,8,9,10,11,12,13,14,15). Yeastolate plays an important role in modern biological production because it can be very effective in promoting insect cell growth and enhancing production of recombinant proteins. The product is known to contain amino acids, peptides, vitamins, minerals, and carbohydrates. It also contains components that have not been characterized; the constituents responsible for cell growth promotion are still a mystery (1,–2).

Yeastolate is cost effective, not animal derived, and can enhance cell growth and recombinant protein production in serum-free culture systems. Yeast cells (Saccharomyces cerevisiae) are expanded for a designated time period, undergo heat shock, and are then autolyzed — or alternatively, they are expanded and then enzymatically hydrolyzed. A filtration process follows, with concentration and ultrafiltration if desired, then finally spray drying (3, 12,13,14,15,16,17). Unfortunately, the activity of yeastolate can vary from lot to lot because of varying fermentation conditions and enzymatic processes. Lots from the same manufacturer can show variability in growth promotion activity. So extensive testing is required to identify favorable lots that will promote cell growth (2).
Because the source and quality of yeastolate can significantly affect insect cell growth and recombinant protein production, efforts have been made to characterize yeastolate and determine its active components (2,–3, 18). Many researchers suspect that small peptides — consisting of six amino acids or fewer — have the greatest effect on cell growth and production (3, 19). So peptide molecular size is one parameter that potentially affects cell performance (1, 20).
The objective of our study was to develop an analytical method to screen incoming yeastolate raw materials and differentiate between acceptable and unacceptable lots. We developed a size-exclusion chromatographic (SEC) method and compared their peptide molecular-weight profile data with cell culture performance testing. We discovered a correlation between these two methods and were able to establish SEC performance specifications.
PRODUCT FOCUS: PROTEINS, BEVS (BACULOVIRUS EXPRESSION VECTOR SYSTEM)
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: ANALYTICAL AND PROCESS DEVELOPMENT
KEYWORDS: INSECT CELLS, CULTURE MEDIA, SUPPLEMENTS, SIZE-EXCLUSION CHROMATOGRAPHY, HPLC
LEVEL: INTERMEDIATE
Materials and Methods
Cell Culture: Sf-21 cells (IPLB-Sf-21-AE) are ovarian cells isolated from Spodoptera frugiperda, the fall army worm (21). The Sf-9 cell line is a clonal isolate derived from a parental Sf-21 cell line. Both are suitable hosts for expression of recombinant proteins using the baculovirus expression vector system (BEVS) (22,23,24). We obtained Sf-9 and Sf-21 cells from Life Technologies in Grand Island, NY. The cells were preadapted to Sf-900 II SFM protein-free insect cell culture medium, which contains yeastolate. It is optimized for growth and maintenance of Sf-9 and Sf-21 cells and for large-scale BEVS production of recombinant proteins. To evaluate Sf-9 and Sf-21 cell response to yeastolate alone, we used Sf-900 II SFM manufactured without yeastolate, added individual lots of yeastolate to it, then subsequently tested that using both Sf-9 and Sf-21 cells.
Stock Cell Maintenance: We maintained Sf-9 stock cells in Sf-900 II SFM and passaged them every three to four days at 2.75–4.00 × 105 viable cells/mL in a 100-mL working volume using 250-mL nonvented Erlenmeyer disposable shake flasks from Corning (Catalog #430183). Similarly, we passaged Sf-21 stock cells every three to four days after seeding them at 2.5–4 × 105 viable cells/mL. All cultures were incubated with loosened caps in a 27 ± 1 °C, non-CO2 Forma Scientific incubator (Model #3919), using an Innova 2100 orbital shaker platform set at 130 RPM.
Assay Establishment: To minimize stock medium carry-over, we washed stock insect cell suspensions in 20 mL of test or control yeastolate assay media by centrifuging them at 130g for four minutes at room temperature. After discarding the supernatants, we resuspended the cell pellets in appropriate test or control yeastolate medium at 3 × 105 viable cells/mL for Sf-9 cells and 2 × 105 viable cells/mL for Sf-21 cells, in a 50-mL working volume using a 125-mL nonvented Erlenmeyer disposable shake flask from Corning (Catalog #430421). We considered this to be assay passage 1.
Four days later, we determined the viable cell counts of all flasks and passaged the cells the same way. But we pelleted them without using a wash medium at 130g for four minutes at room temperature. This time (passage 2), we established triplicate cultures in both test and control assay media. At passage 2, the seeding density remained the same for Sf-9 cells, but it was reduced to 1.5 × 105 viable cells/mL for Sf-21 cells.
The third and final assay passage was four days later for Sf-9 cells and five days later for Sf-21 cells. Triplicate flasks were established using the same Sf-9 and Sf-21 seeding densities as indicated for passage 2. Following passages 2 and 3, we recorded peak viable-cell densities and correlating cell viabilities of all assay flasks and averaged them to calculate the mean viability and peak cell density of all test and control yeastolate media. We considered the performance of test yeastolate media acceptable if it supported growth at ≥90% of yeastolate control media cell density while maintaining a mean insect cell viability of ≥90%.
Cell Counting: We determined all viable-cell densities and cell viabilities in shake flasks using a Vi-Cell XR cell viability analyzer from Beckman Coulter in Jersey City, NJ.
Analysis of Yeastolate Profiles: For size-exclusion chromatography (SEC) analysis of peptides, we used a Tosoh Bioscience TSKgel G2000 SWXL 7.8 mm × 30 cm column purchased from Sigma Aldrich of St. Louis, MO and a solvent
mixture of acetonitrile–H2O–trifluoroacetic acid (30/70/0.1, v/v/v) with an isocratic elution. We set our tunable ultraviolet (TUV) detector at 205 nm. The HPLC system consisted of a Waters 2487 dual-l absorbance detector, 717 plus autosampler, 515 pump. Signal output was captured with Empower 2 chromatography software (Waters Corporation). We had obtained (N-Methyl-Phe3, D-Pro4)-Casomorphin fragment 1-4 amide, Bovine (MW 535) and L-Phenylalanine (MW 165) from VWR International of Radnor, PA.
We determined the molecular size distribution of yeastolate peptides from a calibration curve obtained using peptides of known molar mass as described in the literature (20, 25, 26). As we expected, the values exhibit excellent linearity, with a regression coefficient (R2) of >0.99. Figure 1 shows the standard calibration curve.
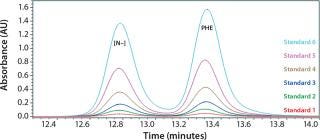
Figure 1: ()
All yeastolate stock solutions were prepared using high-performance liquid chromatography (HPLC) grade water. We prepared each sample by taking 180 µL of a yeastolate stock solution and adding 720 µL of the SEC mobile phase, then vortexing and placing it in an autosampler for analysis.
Results and Discussion
A major concern with the use of yeastolates in cell culture media is their lot-to-lot variability, which can affect both cell growth and recombinant protein production. Cell culture screening of key raw materials such as yeastolate can be laborious. Furthermore, cell culture assays are inherently variable themselves due to stock maintenance methods, techniques, age of cultures, and so on. We believed that a precise, accurate, and timely analytical method would be highly advantageous as a yeastolate screening tool. Not only would it reduce the time needed to qualify this raw material, but it would also help ensure that consistent, high-quality products are being used to manufacture cell culture media.
Testing yeastolate to determine its peptide molecular weight profile can be a screen for qualifying good lots and disqualifying bad lots of this raw material (1, 3). SEC is a good way to obtain those size profiles.
To determine what peptide markers to use for a calibration curve to match the particular retention times of yeastolate components, we injected several known markers individually and determined their retention times. Figure 2 shows an overlay of several known such markers with their retention times. After some test injections of yeastolate, we eliminated several markers from the analysis and determined that two remaining markers would be sufficient. After further development work, we established an optimal peak shape for (N-Methyl-Phe3, D-Pro4)-Casomorphin fragment 1-4 amide, Bovine (MW 535) and L-Phenylalanine (MW 165).
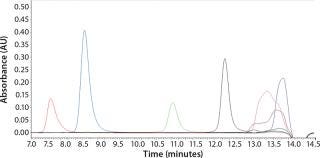
Figure 2: ()
Initial SEC testing revealed differences in chromatography profiles among several yeastolate vendors as well as lot-to-lot sample variability for each supplier (Figure 3). We implemented known peptide molecular weight markers and their retention times as guidelines for raw-material qualification using SEC. The raw-material chromatogram was divided into several areas, each pertaining to a different known peptide molecular weight. We deemed yeastolates to be acceptable when the first section of their chromatograms’ percent area of total was ≤11% and the second section’s percent area of total was ≤28%. Both criteria had to be met.
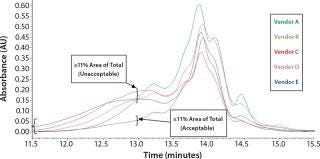
Figure 3a: ()
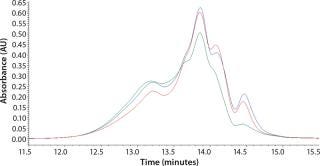
Figure 3b: ()
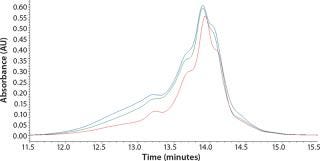
Figure 3c: ()
Our studies verified that the SEC assay correlated significantly with the cell culture performance assay. In Figure 4, media containing yeastolate sample lots 1–4 demonstrated acceptable Sf-9 and Sf-21 growth, meeting the minimum acceptance criterion of ≥90% of the yeastolate control cell density. The same samples maintained a mean insect cell viability of ≥90% (data not shown). Those sample lots also met the peptide molecular weight criterion. Yeastolate sample lots 5–7 met the growth requirement for Sf-9 cells (blue bars), but not for Sf-21 cells (red bars). Finally, yeastolate sample lots 8–13 failed to demonstrate acceptable cell growth for either insect cell line. In correlation, yeastolate sample lots 5̵
1;13 failed to meet the peptide molecular weight profile criterion; these samples had higher percentages of larger molecular weights than those of the passing lots.
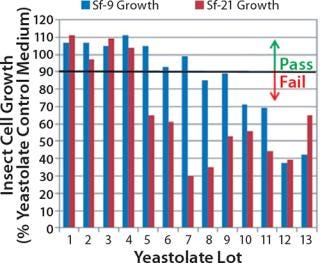
Figure 4: ()
Analytical metrics of the SEC method include repeatability, specificity, accuracy, precision, linearity, limits of detection, and quantification. The calibration curves were linear, with average correlation coefficients (R2) for (N-Methyl-Phe3, D-Pro4)-Casomorphin fragment 1-4 amide, Bovine (MW 535) and L-Phenylalanine (MW 165) at 0.999 (0.000 standard deviation, SD) and 0.999 (0.001 SD) calculated from 10 calibration curves. Intraday precision values for (N-Methyl-Phe3, D-Pro4)-Casomorphin fragment 1–4 amide, Bovine (MW 535) and L-Phenylalanine (MW 165) were 0.080% and 0.268%. Intraday accuracy values for (N-Methyl-Phe3, D-Pro4)-Casomorphin fragment 1–4 amide, Bovine (MW 535), and L-Phenylalanine (MW 165) were 98.1% and 94.0%.
We analyzed our raw HPLC data by integrating the extracted SEC chromatograms for each peptide. Instrument repeatability was determined to be 0.34% and 0.17%. We integrated the raw data for each sample into three areas represented by retention times of known peptide markers. This SEC assay is able to qualify yeastolate raw material much faster than the traditional cell culture performance assay.
By screening key incoming raw materials such as yeastolate with such fast analytical methods, companies could omit some traditional, laborious cell culture screening assays. This would provide for better lot-to-lot consistency of final cell culture media to enable reliable Sf-9 and Sf-21 cell growth and recombinant protein production. From a business perspective, use of the SEC screening method described herein improves the characteristics of yeastolate used in cell culture media manufacturing, which enables suppliers to produce high-quality cell culture media.
About the Author
Author Details
Corresponding author Kim M. Keil, PhD, and Mary Lynn Tilkins are staff scientists in manufacturing science at Life Technologies, 3175 Staley Road, Grand Island, NY 14072; [email protected]; www.lifetech.com.
REFERENCES
1.) Deparis, V. 2003. Promoting Effect of Rapeseed Proteins and Peptides on Sf9 Insect Cell Growth. Cytotechnol. 42:75-85.
2.) Shen, CF. 2007. Characterization of Yeastolate Fractions That Promote Insect Cell Growth and Recombinant Protein Production. Cytotechnol. 54:25-34.
3.) Dick, LW. 2009. Investigation of Proteins and Peptides from Yeastolate and Subsequent Impurity Testing of Drug Product. Biotechnol. Progr. 25:570-577.
4.) Ikonomou, L, Y-J Schneider, and SN. Agathos. 2003. Insect Cell Culture for Industrial Production of Recombinant Proteins. Appl. Microbiol. Biotechnol. 62:1-20.
5.) Aucoin, MG. 2007. Virus-Like Particle and Viral Vector Production Using the Baculovirus Expression Vector System/Insect Cell System: Adeno-Associated Virus-Based Products. Meth. Mol. Biol. 388:281-296.
6.) Loane, M. 2007. The Potential of Capillary Electrophoresis for the Analysis of Yeast Extracts, Dublin City University, Dublin.
7.) Wu, J, and KD. Lee. 1998. Growth Promotion By Yeastolate and Related Components on Insect Cells. Biotechnol. Techniq. 12:67-70.
8.) Agathos, SN. Murhammer, DW 2007.Development of Serum-Free Media for Lepidopteran Insect Cell Lines. Methods in Molecular BiologyBaculovirus and Insect Cell Expression Protocols, Humana Press, New York:155-185.
9.) Sung, YH. 2004. Yeast Hydrolysate as a Low-Cost Additive to Serum-Free Medium for the Production of Human Thrombopoietin in Suspension Culture of Chinese Hamster Ovary Cells. Appl. Microbiol. Biotechnol. 63:527-536.
10.) Schär-Zammaretti, P. 2005. Influence of Fermentation Medium Composition on Physicochemical Surface Properties of Lactobacillus acidophilus. Appl. Environ. Microbiol. 71:8165-8173.
11.) Song, H, and SY. Lee. 2006. Production of Succinic Acid By Bacterial Fermentation. Enz. Microb. Technol. 39:352-361.
12.) Donaldson, MS, and ML. Shuler. 1998. Low-Cost Serum-Free Medium for the BTI-Tn5B1-4 Insect Cell Line. Biotechnol. Progr. 14:573-579.
13.) Ikonomou, L. 2001. Design of an Efficient Medium for Insect Cell Growth and Recombinant Protein Production. In Vitr. Cell Biol. 37:549-559.
14.) Nguyen, B. 1993. Fed-Batch Culture of Insect Cells: A Method to Increase the Yield of Recombinant Human Nerve Growth Factor (rhNGF) in the Baculovirus Expression System. J. Biotechnol. 31:205-217.
15.) Bedard, C. 1994. Maximization of Recombinant Protein Yield in the Insect Cell/Baculovirus System By One-Time Addition of Nutrients to High-Density Batch Cultures. Cytotechnol. 15:129-138.
16.).
17.) Franek, F. 2000. Plant Protein Hydrolysates: Preparation of Defined Peptide Fractions Promoting Growth and Production in Animal Cell Cultures. Biotechnol. Progr. 16:688-692.
18.) Mendonca, RZ. 2007. Effect of Bioactive Peptides Isolated from Yeastolate, Lactalbumin, and NZCase in the Insect Cell Growth. Biopr. Biosyst. Eng. 30:157-164.
19.) Franek, F, and M. Fussenegger. 2005. Survival Factor-Like Activity of Small Peptides in Hybridoma and CHO Cell Cultures. Biotechnol. Prog. 21:96-98.
20.) Farges-Haddani, B. 2006. Peptide Fractions of Rapeseed Hydrolysates as an Alternative to Animal Proteins in CHO Cell Culture Media. Proc. Biochem. 41:2297-2304.
21.) Vaughn, JL. 1977. The Establishment of Two Cell Lines from the Insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro 13:213-217.
22.) Smith, GE, MD Summers, and MJ. Fraser. 1983. Production of Human Beta Interferon in Insect Cells Infected with a Baculovirus Expression Vector. Mol. Cell. Biol. 3:2156-2165.
23.) Pennock, GD, C Shoemaker, and LK. Miller. 1984. Strong and Regulated Expression of Escherichia coli Beta-Galactosidase in Insect Cells with a Baculovirus Vector. Mol. Cell. Biol. 4:399-406.
24.) Jarvis, DL. Miller, LK 1997.Baculovirus Expression VectorsThe Baculoviruses, Plenum Press, New York:389-431.
25.) Tessier, B. 2006. Selective Separation of Peptides in a Rapeseed (Brassica campestris L.) Protein Hydrolysate Using UF/NF Membrane. J. Agric. Food Chem. 54:3578-3584.
26.) Tessier, B. 2005. Prediction of the Amino Acid Composition of Small Peptides Contained in a Plant Protein Hydrolysate By LC-MS and CE-MS. Food Res. Int. 38:577-584.
You May Also Like





