Pressure Vessels for Biomanufacturing: Basic Considerations for Cleaning and Process CompatibilityPressure Vessels for Biomanufacturing: Basic Considerations for Cleaning and Process Compatibility
June 21, 2022
Pressure vessels are enclosed containers used to contain liquids, vapors, and gases at pressures that are significantly higher or lower than the ambient pressure of their surroundings. Equipment such as bioreactors, holding tanks, mixing tanks, separators, and heat exchangers all are examples of pressure vessels. As such, they form an integral part of biopharmaceutical manufacturing. Apart from pressure containment itself, a key challenge in building pressure vessels is making them meet the high purity and cleanability requirements of bioprocessing.
As single-use technologies have been integrated into more bioprocess operations over the past couple of decades, their suppliers have addressed many associated concerns such as those related to scale and leachables/extractables, as well as supply-chain and waste questions that arose with expanded use of disposables. Even the gassing rate, mixing power, and heat removal needs of microbial fermentation applications are beginning to be met now by some single-use fermentors. Pressure remains perhaps the most challenging issue for polymer-based components and assemblies, as anyone who has dealt with leaking bags can tell you. Thus, even as the biopharmaceutical industry transitions toward manufacturing in single-use systems, the need for hard-piped metal pressure vessels remains for many processes and the facilities that house them. In fact, such technologies still dominate the majority of bioprocess facilities today, especially for operations with manufacturing scales larger than 4,000 L.
Material Selection Criteria
The materials used in construction of pressure vessels for biopharmaceutical manufacturing are limited compared with those for other industries (e.g., specialty chemicals). Before choosing a suitable material for a given application, it is important to note that such material must meet specific criteria according to good manufacturing practice (GMP) (1):
• “Equipment shall be constructed so that surfaces that contact components, in-process materials, or drug products shall not be reactive, additive, or absorptive so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.”
• “Any substances required for operation, such as lubricants or coolants, shall not come into contact with components, drug product containers, closures, in-process materials, or drug products so as to alter the safety, identity, strength, quality, or purity of the drug product beyond the official or other established requirements.”
It also is important for materials to be fit for their intended purpose of fluid service. They must be able to meet the design and operating conditions (pressure/temperature) for a given equipment design. And they need to withstand clean-in-place (CIP) or steam-in-place (SIP) regimens used for cleaning such equipment.
The most common construction materials for pressure vessels used in the biopharmaceutical industry are 300-series stainless steel, glass-lined carbon steel, superaustenitic stainless steels, duplex stainless steel, nickel-based alloys, and titanium. Originally designed for seawater resistance, AL-6XN (Rolled Alloys, Inc.) and 904L superaustenitic stainless steels also are resistant because of their molybdenum content to a broad range of corrosive environments. Duplex stainless steels 2205 and 2507 are nitrogen-enhanced for applications requiring high strength as well as corrosion resistance. Nickel-based alloys from are resistant to corrosion, oxidation, and high temperatures.
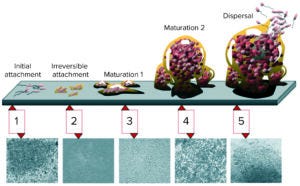
Figure 1: Biofilm maturation is a complex, five-stage developmental process (2).
Surface Finish Matters: The surface finish of product/process contact areas is an important aspect of biopharmaceutical vessels. Rough surfaces promote the growth of bacteria by providing them with the means to cling on and gradually form biofilms (Figure 1). Figure 1 shows the five stages of biofilm maturation, each stage of development paired with a photomicrograph of a developing Pseudomonas aeruginosa biofilm (2). The bacteria form colonies inside surface pits and crevices, creating bioburden as their counts increase, and the rough surface both protects them and promotes their growth. It is difficult to reach and clean such areas, a limitation that ultimately can result in contamination of manufacturing batches.
Cleaning Concerns
Equipment cleaning is important to bioburden control. The best way to clean a pressure vessel is to fill it up completely with an appropriate cleaning solution, then hold it for a set period based on the vessel size before draining it fully, followed by rinsing with water for injection (WFI). Such procedures are not always possible once equipment is in place and in service, however. That’s where CIP comes into use for cleaning equipment internally without disassembly, but the technique brings its own difficulties.

Photo 1: A typical spray ball.
Spray balls (Photo 1) and other devices used for such cleaning come from several suppliers in a number of configurations (e.g., static, rotary, and rotary jet types). The choice of which one to use depends on an application and its required pressure and flow rates. Before finalizing the decision for a particular use, a vessel must be evaluated for maximum cleaning-solution coverage without shadow areas inside. Not every spray ball will provide full coverage for every vessel design. Thus, CIP coverage studies are always advisable (3–7).
Shadow areas are hard-to-reach sections of a vessel that cleaning solution cannot reach through spraying. Examples include areas inside nozzle necks, near internal attachments, and underneath agitator blades. A riboflavin test helps to ascertain the coverage area inside a vessel (7–10). Riboflavin liquid glows under UV light to show the coverage area. If any area lacks coverage, then either the chosen spray ball must be modified accordingly or a new model should be selected to provide complete coverage.
Cleanability and removability of individual spray balls must be ensured. CIP coverage studies also investigate the following parameters:
• cleaning solvent coverage
• spray-ball location for complete coverage
• shadow areas to consider in selecting spray ball model and size
• cleaning solvent quantities and number of cleaning cycles
• pressure and flow rates for each potential spray ball.
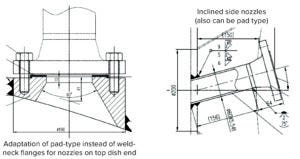
Figure 2: Configurations for complete spray coverage.
Design Improvements for Complete CIP Coverage: Pad flanges can replace weld-neck flanges for design of nozzles on the top dish end of some vessels (Figure 2). Pad flanges provide the shortest possible projection both outside and inside a vessel, which can be important for mounting certain equipment. For example, operators and engineers often need to see inside a vessel during certain processes.
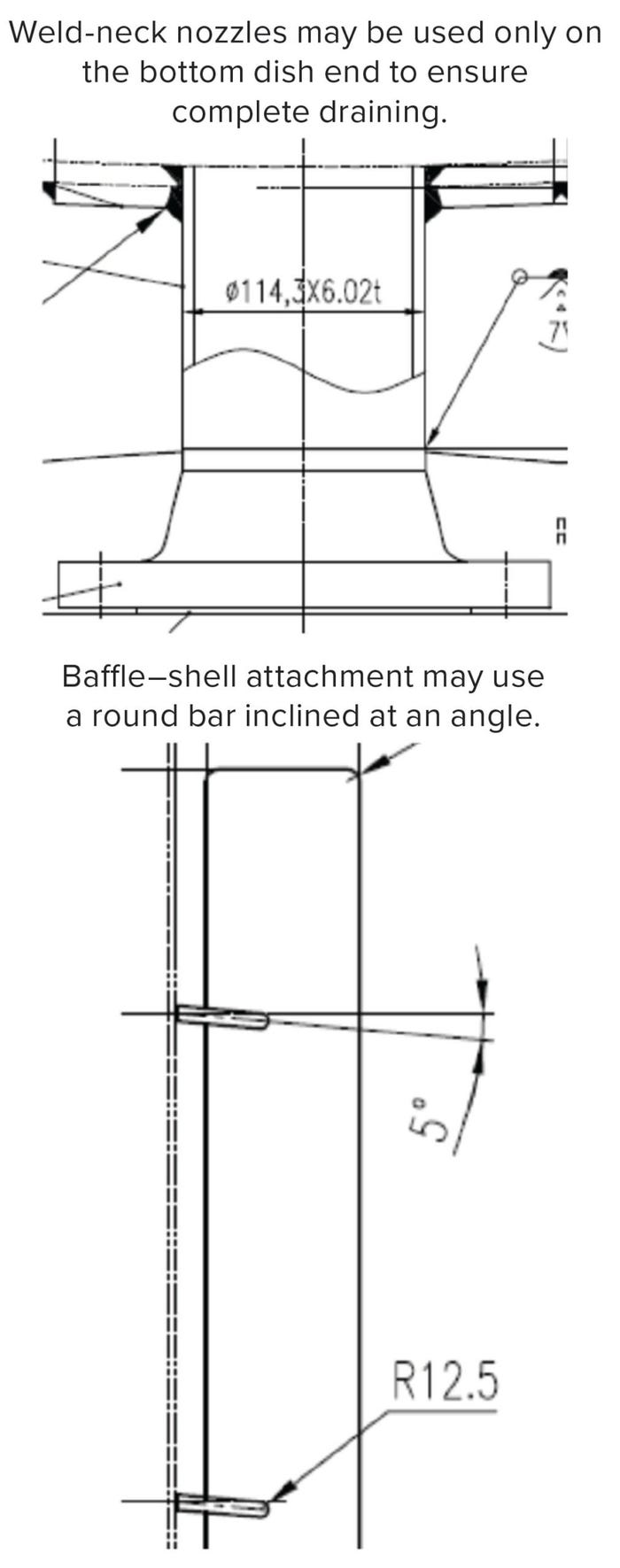
Figure 3: Configurations for complete drainability.
Inclined side nozzles (Figure 2) help to distribute even spray coverage. Weld-neck nozzles can be used on the bottom dish end of a vessel to ensure complete drainage. Some applications might require attachment of a draining baffle to the vessel shell, for which a round bar inclined at an angle can be considered.
All internal attachments should be fillet curved to facilitate complete drainage without any deadlegs (areas that trap and retain materials such as cleaning and process fluids). Common to welded, soldered, and brazed joints, fillet geometry creates a line of concave function inside a vessel or a convex corner (a round) on the outside. Finally, if bottom outlet valves are to be used, then they must be completely drainable and cleanable without disassembly.
Rouging
Rouge presents as a discoloration of high-purity stainless steel in biopharmaceutical systems caused by corrosion products that are composed of iron oxides. Based on its source, rouge is categorized into three types. Type I comes from upstream corrosion; types II and III come from the site where they appear.
Type I Rouging: Iron oxides are generated by erosion or cavitation of external surfaces. They can be attached to a vessel surface through electrostatic attraction. The stainless-steel surface under such oxides generally is unaffected, with no corrosion. These brownish, easy-to-clean oxides have the chemical composition of their “upstream” originating source (e.g., a pump impeller).

Photo 2: Example of type II rouging inside a pressure vessel.
Type II Rouging: In situ oxidation of stainless steel tightly adheres to a vessel’s surface and damages the passive layer. This difficult-to-clean rouging appears reddish brown. The surface below it usually is pitted by the presence of chlorides and halides.
Type III Rouging: Black oxide rouges usually manifest in high-temperature steam-service vessels. Very difficult to clean (requiring specialized chemical cleaning agents), this rouging is durable and will accelerate surface damage over time.
Stick to the Standards
The primary purpose of a pressure vessel is containment of pressure inside it — whether higher or lower than the ambient pressure surrounding it — to meet the stringent requirements of the American Society of Mechanical Engineers (ASME) boiler and pressure vessel code (BPVC) section VIII (11). But when it comes to pressure vessel design for high-purity applications such as biopharmaceutical processing, other requirements for design, fabrication, and material also must be met (12). A properly designed vessel should perform exceptionally without causing product contamination, which is paramount in biomanufacturing.
References
1 Section 211.65: Equipment Construction. Code of Federal Regulations Title 21, Chapter 1, Subchapter C, Subpart D. US Food and Drug Administration: Rockville, MD, 29 March 2022 update; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=211.65.
2 Monroe D. Looking for Chinks in the Armor of Bacterial Biofilms. PLoS Biol 5(11) 2007: e307; https://doi.org/10.1371/journal.pbio.0050307.
3 Mollah AH. Cleaning Validation for Biopharmaceutical Manufacturing at Genentech, Inc. Part 1. BioPharm Int. 21(2) 2008: https://www.biopharminternational.com/view/cleaning-validation-biopharmaceutical-manufacturing-genentech-inc-part-1.
4 Mollah AH. Cleaning Validation for Biopharmaceutical Manufacturing at Genentech, Inc. Part 2. BioPharm Int. 21(3) 2008: https://www.biopharminternational.com/view/cleaning-validation-biopharmaceutical-manufacturing-genentech-inc-part-2.
5 Roy K, et al. Multivariate Statistical Monitoring As Applied to Clean-in-Place (CIP) and Steam-in-Place (SIP) Operations in Biopharmaceutical Manufacturing. Biotechnol. Prog. 30(2) 2014: 505–515; https://doi.org/10.1002/btpr.1880.
6 Clean-In-Place for Biopharmaceutical Processes. Seiberling DA, Ed. CRC Press: Boca Raton, FL, 2008.
7 Wiencek M. Biotech CIP Cycle Development: Case Study Examples Utilizing QRM. Pharm. Eng. 26(5) 2006; https://ispe.org/sites/default/files/attachments/public/Sept-Oct-2006.pdf.
8 Mayo J. Validating Reactor Cleanability with Clean-In-Place (CIP) Studies. DeDietrich Process Systems Blog 24 October 2019; https://www.ddpsinc.com/blog/validating-reactor-cleanability-with-clean-in-place-cip-studies.
9 Verghese G , Lopolito P. Cleaning Engineering and Equipment Design. Cleaning and Cleaning Validation. Pluta P, Ed. PDA & DHI Publishing: Scottsdale, AZ, 2009.
10 LS432EN. Riboflavin Coverage Test. Steris Life Sciences: Mentor, OH, 1 December 2020; https://www.sterislifesciences.com/-/media/files/lifesciences_com/pdf/tech-lab/riboflavin-coverage-test.ashx.
11 BPVC-VIII-1-2021. BPVC Section VIII-Rules for Construction of Pressure Vessels, Division 1. ASME: New York, NY, 2021; https://www.asme.org/codes-standards/find-codes-standards/bpvc-viii-1-bpvc-section-viii-rules-construction-pressure-vessels-division-1/2021/print-book.
12 BPE-2019. Bioprocessing Equipment. ASME: New York, NY, 2019; https://www.asme.org/codes-standards/find-codes-standards/bpe-bioprocessing-equipment-(1).
Further Reading
Baccarelli C, et al. Cleaning of Dedicated Equipment: Why Validation Is Needed. BioPharm Int. 28(8) 2015; https://www.biopharminternational.com/view/cleaning-dedicated-equipment-why-validation-needed-0.
Boulanger R, et al. Shared Clean-in-Place Systems: To Share or Not to Share? BioProcess Int. 18(4) 2020: 16–22; https://bioprocessintl.com/manufacturing/facility-design-engineering/shared-clean-in-place-systems-to-share-or-not-to-share.
Cardot JM, Beyssac E. The Basics of Cleaning and Cleaning Validation. BioProcess Int. 5(6) 2007: 66–76; https://bioprocessintl.com/analytical/downstream-validation/the-basics-of-cleaning-and-cleaning-validation-60120078.
Embury M. Where Stainless Steel Fits In. Pharma Manuf. 2 March 2020: https://www.pharmamanufacturing.com/articles/2020/where-stainless-steel-fits-in.
Gonzalez MM. Rouge: The Intrinsic Phenomenon in 316L Stainless Steel — a Key Material in Biopharmaceutical Facilities. Pharm. Eng. 32(4) 2012; https://www.bpe-technology.com/system/download/Rouging%20in%20Stainless%20Steel%20(Pharmaceutical%20Engineering%202012).pdf.3d0d924a8ca6f7b5969a6eaea9bf27aa.
Guide to Bio/Pharma Engineering Services: Vessel and Tank Design for Demanding Bio/Pharma Processing Applications. Lee Industries: Phillipsburg, PA, 2017; https://innovation.leeind.com/acton/media/24962/lee-industries-bpdps-bio-pharma-12-17.
Johnston R. The Dinosaurs Reborn: Evaluating Stainless Steel and Disposables in Large-Scale Biomanufacturing. BioProcess Int. 8(12) 2010: 28–33; https://bioprocessintl.com/upstream-processing/upstream-single-use-technologies/the-dinosaurs-reborn-evaluating-stainless-steel-and-disposables-in-large-scale-biomanufacturing-307212.
Langer E, Rader R. Biopharmaceutical Manufacturing Is Shifting to Single-Use Systems: Are the Dinosaurs, the Large Stainless Steel Facilities, Becoming Extinct? Am. Pharm. Rev. 23 October 2018: https://www.americanpharmaceuticalreview.com/Featured-Articles/354820-Biopharmaceutical-Manufacturing-is-Shifting-to-Single-Use-Systems-Are-the-Dinosaurs-the-Large-Stainless-Steel-Facilities-Becoming-Extinct/.
Langer E, Rader R. Innovation In Stainless-Steel Bioprocessing. Life Sci. Leader 31 October 2013: https://www.bioprocessonline.com/doc/innovation-in-stainless-steel-bioprocessing-0001.
Langer ES. Advances in Bioprocessing Single-Use and Stainless Steel Technologies. BioProcess Int. 13(11) 2015: S2–S5; https://bioprocessintl.com/manufacturing/facility-design-engineering/advances-in-bioprocessing-single-use-and-stainless-steel-technologies.
Lopolito P. Addressing Rouge in Biopharmaceutical Manufacturing Systems. Pharm. Technol. 14 September 2020: https://www.pharmtech.com/view/addressing-rouge-biopharmaceutical-manufacturing-systems.
Mietzner MW. Equipment Surfaces: Redefining Acceptable Conditions to Eliminate Unnecessary Maintenance. Pharm. Eng. November–December 2018: https://ispe.org/pharmaceutical-engineering/november-december-2018/equipment-surfaces-redefining-acceptable.
Rivera E, Lopolito P, Hadziselimovic D. A Risk-Based Approach to Stainless Steel Equipment Maintenance. Pharm. Technol. 41(2) 2017; https://www.pharmtech.com/view/risk-based-approach-stainless-steel-equipment-maintenance.
Rogge P, Müller D, Schmidt SR. The Single-Use or Stainless Steel Decision Process: A CDMO Perspective. BioProcess Int. 13(11) 2015: S10–S15; https://bioprocessintl.com/manufacturing/single-use/the-single-use-or-stainless-steel-decision-process-a-cdmo-perspective.
Sandle T. Risk Considerations for Aging Pharmaceutical Facility Cleanrooms. BioProcess Int. 19(5) 2021: 10–13; https://bioprocessintl.com/manufacturing/facility-design-engineering/risk-considerations-for-aging-pharmaceutical-facilities-and-cleanrooms.
Scott C. Large-Scale Capacity Strategies: Single Use, Multiuse, or Both? BioProcess Int. 17(6) 2019: i1–i4; https://bioprocessintl.com/manufacturing/facility-design-engineering/large-scale-capacity-strategies-single-use-multiuse-or-both.
Suthan Rupesh is a senior mechanical engineer with PM Group, Killakee House, Belgard Square W, Tallaght, Dublin, D24 XFW2, Ireland; 353-1-404-0700; [email protected].
You May Also Like





