Advantages of Archived Standard Curves for the Quantitative Bacterial Endotoxins TestAdvantages of Archived Standard Curves for the Quantitative Bacterial Endotoxins Test
July 1, 2009

Figure 1:
The bacterial endotoxins test (BET) is required for most parenteral drugs and medical devices that come into contact with the human bloodstream. Limulus amebocyte lysate (LAL) derived from horseshoe crabs is used for the BET, and several methodologies have been developed. Popular quantitative LAL techniques are the kinetic turbidimetric assay (KTA) and the kinetic chromogenic assay (KCA). It is, however, well known that the endotoxin standards used in both those assays are profoundly influenced by the techniques and materials used by trained LAL analysts each and every day. The type of pipettes used, the type of dilution tubes used, the length of time used to vortex endotoxin standards, and the stability of the liquid stock endotoxin solution are variables that laboratory personnel manage every day. The Portable Test System (PTS) is a new FDA-approved KCA method that uses an archived standard curve. Here, advantages of the archived standard curve method are demonstrated and discussed.
Materials and Methods
A lyophilized positive endotoxin sample lot (positive sample, PS) was prepared with mannitol and sodium chloride in single-test vials. The potency of PS was about 0.2–0.3 EU/vial, and its vial-to-vial reproducibility was <7%. The potency of the positive samples was determined for 16 separate times by KCA with Endochrome-K (lot X4512E, Charles River) on a microplate reader, KTA with a gel-clot LAL (lot W3411L, Charles River) on a tube reader, and PTS (lot 9462153, Charles River) on a PTS reader. Standard curves were prepared every day for KCA and KTA, and an archived standard curve by the manufacturer was used for PTS. The averaged measured values of PS for each of the techniques were calculated and recorded as 100% sample value. The individual measured values of PS were converted to percentages by using the averaged values as 100%.
Results and Discussion
As shown in Figure 1, PTS using the archived standard curve method showed comparable results with other quantitative techniques. All measured values of PS were in the range of 80% and 120% except one with KTA (Run# 7).
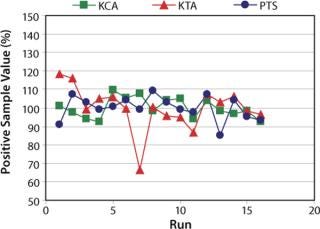
Figure 1: ()
There was an accidental measurement with KTA in Run# 7. The measured value was 66% of the averaged value. Although the value was in the acceptance range of 50% and 200%, the value was obviously lower than other replicates. The operator realized the error after the measurement because the onset times of the standard dilutions were different from those in previous measurements. He investigated the procedures of the measurement and found that the volume for the preparation of the standard dilutions was incorrect. Since he knew the trend of the onset times of the standard dilutions and was careful enough to realize the difference, he could find the error. However, it is not possible to find this type of error if the LAL reagent lot is new and the operator is not observant.
An archived standard curve for KTA was established by using averaged onset times at each concentration of standard dilution. The concentrations of PS were recalculated with the archived standard curve. The recalculated values are more consistent than the original values calculated with only the daily standard curves, as shown in Figure 2. This indicates that the archived standard curve method, which is employed on all PTS systems, can eliminate accidental errors during the preparation of daily standard dilutions in the LAL test.
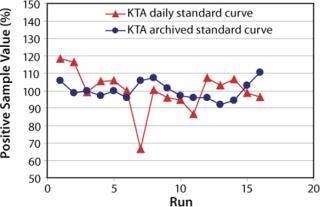
Figure 2: ()
Conclusion
Conventional quantitative LAL techniques, such as KCA and KTA are under the influence of the daily standard curves, each having potentially variable bias. By contrast, the PTS uses an archived standard curve, which gives a constant bias. The use of multiple replicates to create the archived standard curve and the long term experience of the manufacturer’s QC technicians minimizes the opportunity for problems. The archived standard curve method is useful to avoid the errors that occur during the preparation of daily endotoxin standard dilutions which are seldom realized by operators.
REFERENCES
1.) 1987.Guideline on Validation of the Limulus Amebocyte Lysate Test as an End-Product Endotoxin Test for Human and Animal Parenteral Drugs, Biological Products, and Medical Devices, US Food and Drug Administration, Rockville.
2.) 1991.Interim Guidance for Human and Veterinary Drug Products and Biologicals, Kinetic LAL Techniques, US Food and Drug Administration, Rockville.
3.) Wainwright, NR, D Nutter, and F. Jordan Miller, MJ.. 2005.Encyclopedia of Rapid Microbiological Methods, PDA, Bethesda:97-119.
You May Also Like






