Biases in the Bacterial Endotoxin TestBiases in the Bacterial Endotoxin Test
July 1, 2010

The bacterial endotoxins test (BET) is unique because of its biases. Several biases in the BET are caused by the variability of standard endotoxin dilutions and the methodologies of the Limulus amebocyte lysate (LAL) test. The purpose of this study is to clarify those biases.
Bias from the Standard Endotoxin Dilutions
Operators for the BET often encounter variability of the potency in the standard endotoxin dilutions. To clarify the practical variability of the potency in the reference standard endotoxoin (RSE) dilutions, analysis was performed of the daily potency check in a Charles River laboratory. The operators confirm the potency of the RSE dilutions daily prepared for the kinetic turbidimetric assay (KTA) before use. The averages of the onset times at each endotoxin concentration were calculated. The total number of the measurement was 174 during the period between 10 September 2008 and 5 June 2009. An average standard curve was established using the average onset times and endotoxin concentrations between 0.5 and 0.03 EU/mL (five concentrations). Individual endotoxin concentrations were recalculated using the average standard curve from the individual onset times. The ratios of individual endotoxin concentrations against the average endotoxin concentrations were calculated for each assay. The average ratio of each assay was calculated using ratios of the five concentrations of endotoxin in each assay and was used as the average potency ratio. The average of the potency ratios of each RSE vial was also calculated.
Figure 1 shows the variability of the potency of the RSE dilutions. The range of the individual potency was between 62% and 147%. This is within the acceptable range of 50% and 200%. There was significant difference in the average potency between the RSE vials by the Kruskal-Wallis Test (p = 0.0105).
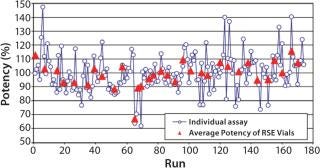
Figure 1: ()
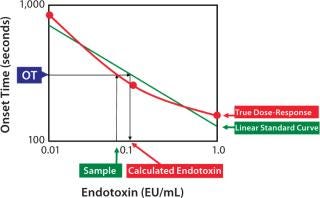
Figure 2: ()
If the endotoxin standard shows consistent potency, daily standard curves will give most accurate results. However, potency of standard endotoxin dilutions is not very consistent because of the errors in preparation, vial-to-vial variability of the standard endotoxin, and potency change of the endotoxin dilutions. The results of the daily potency check of the RSE dilutions by well-trained operators demonstrated that the variability of the potency was between 60% and 150%. This range may vary due to personal and environmental reasons. This bias that cannot be realized by the operators affects all kinds of the LAL methods. Higher and lower potency of RSE dilutions gives underestimation and overestimation in the endotoxin assay, respectively.
Conclusions
Biases in the LAL test are caused by variability in the potency of the RSE dilutions and biases specific in the methodologies of the test. The bias in the gel-clot method gives overestimation. Biases specific in kinetic LAL methods are caused by the shape of the standard curves. Accidental potency difference of the RSE dilutions affects results in the kinetic LAL methods instead of the PTS™ method. The PTS™ method is robust against the accidental potency difference of the RSE dilutions because of the use of the archived standard curves.
About the Author
Author Details
Masakazu Tsuchiya, PhD, is in endotoxin and microbial detection at Charles River Laboratories International, Inc., 251 Ballardvale Street, Wilmington, MA 01887; 1-781-222-6000; [email protected], www.criver.com.
You May Also Like






