Bio on Demand August 2012Bio on Demand August 2012
August 1, 2012

NNE Pharmaplan has developed a new facility concept we call Bio on demand™. Facilities based on the Bio on demand™ concept can be established in one to two years. The result is a flexible facility that is fully operational, locally compliant, and with functioning quality systems. The Bio on demand™ concept involves a high degree of single-use technology to ensure cost-effective production and fast establishment.
Facilities based on our Bio on demand™ concept can be delivered globally, but they are primarily intended for growth markets where time-to-market is particularly essential. We are currently applying the standard Bio on demand™ concept in the design of a number of new biotech facilities for both local and international companies established in emerging markets.
Full Package for Full Predictability
The Bio on demand™ concept includes engineering and supply of a facility as well as related quality systems, standard operating procedures (SOPs) and coordination of necessary quality tests. By not only providing a facility, but a full turn-key solution, we allow our customers to focus on creative aspects rather than technicalities. Also the customer gains better predictability of the project and future production.

Facilities of the Future: A next-generation biopharmaceutical facility concept designed for global scalability and inspired by innovation ()
High Flexibility for Customised Solutions
Bio on demand™ is a standard concept that can be adapted to individual customers’ needs and for local and site-specific conditions. Based on our Bio on demand™ facility concept we can build facilities on site in the traditional way or off site as a modular facility, depending on the customer’s preferences. Standardised process and utility modules are combined in various ways to accommodate all the functions in a modern biotech facility and meet the need for flexibility and adaption to local building and GMP regulations and practices. The concept is adaptable for a wide range of business cases and commercial possibilities.
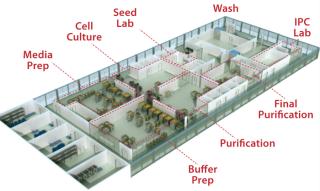
Figure 1: ()
Accelerated Timeline for Speed to Market
Our Bio on demand™ concept enables fast-track project execution with parallel execution and single-use technology that decouples building and process to allow late freeze of process. Verification is based on ASTM E-2500, which ensures speed and cost-effectiveness. The overall focus is on integration of activities related to first GMP batch for a timeline target of only 18 months from finished basic design to the start of the first GMP batch.
Who We Are
NNE Pharmaplan is the world’s leading engineering and consulting company in the complex field of pharma and biotech. We cover all segments from biopharmaceuticals and vaccines to medical devices and help our customers develop, establish, and improve their product manufacturing. NNE Pharmaplan employs 1,700 people at more than 30 locations around the world.
To learn more about our company and our Bio on demand™ facility concept, please visit nnepharmaplan.com.
About the Author
Author Details
Niels Guldager is senior technology partner of biopharmaceuticals at NNE Pharmaplan, Nybrovej 80, 2820 Gentofte Denmark, 45-3079-7246; [email protected].
You May Also Like






