Outsourcing GMP BiologicalsOutsourcing GMP Biologicals
July 1, 2010

Figure 1: ()
Selection of a CMO is critical to biologic drug development; and the primary selection criterion is experience. How do biotech companies measure experience? CMO experience can be measured in many ways — primary metrics include (1) total number of GMP processes developed by a CMO; (2) number of projects involving your strain; (3) number of projects involving your product type; (4) GMP production success rate; (5) total number of GMP lots produced; and (6) number of quality audits.”
Eurogentec’s biologics division is specialized in the manufacturing of biopharmaceuticals from microbial sources: bacteria, yeast, and biosafety level 2 (BSl-2) organisms. Eurogentec has manufactured according to GMP since 1994.
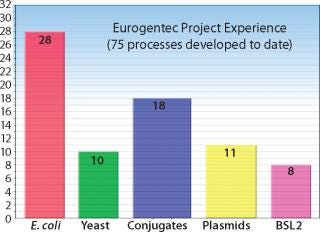
Figure 1: ()
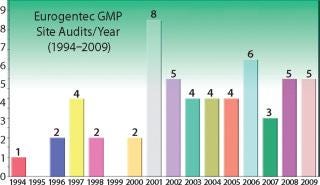
Figure 2: ()
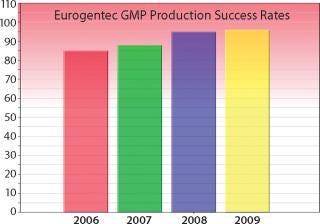
Figure 3: ()
Host Cell Experience: Microbial Experts
Eurogentec has expertise with E. coli, P. pastoris, H. polymorpha, S. cerevisiae, and numerous BSl-2 organisms.
Chemistry Experience: Conjugation Experts
Maleimide
Glutaraldehyde
Other chemistries available
Product Family Experience
Recombinant proteins
Plasmid DNA
Protein-PEG conjugations
Protein-protein conjugations
Protein-peptide conjugations
GMp Infrastructure
Multiproduct facility
Separate HVAC systems
GMP fermentation suites: 80 L, 150 L, 500 L fermenters
2 GMP purification suites
1 GMP sterile filtration suite
Process Development Strategies
FastTrack
OptiTrack
About the Author
Author Details
Dr. Pascal Bolon is biologics sales and marketing manager at Eurogentec SA, Rue du Bois Saint-Jean 5, 4102 Seraing, Belgium; +32 4- 366-6116; [email protected].
You May Also Like






