Training for New and Emerging Technologies: Building Comprehensive, Process-Oriented CompetenciesTraining for New and Emerging Technologies: Building Comprehensive, Process-Oriented Competencies
Assessing the need for training that addresses the varied needs of your employees requires looking far beyond a one-size-fits-all approach. Few companies, however, have the internal resources to develop truly comprehensive programs on their own. Successful training approaches are those that enable end-users, organizations, and suppliers to share needed equipment and expertise.
At the 2016 BioProcess Theater at Interphex, Gary Gilleskie (BTEC’s director of operations) and Scott Sommer (a technical fellow at Renmatix) detailed examples of successful training programs for new and emerging technologies. Below, Gilleskie describes BTEC’s successful approach for training in single-use technologies, and Sommer explains the importance of improving the quality of and access to automation training and existing certification programs.

Photo 1: Short course participants learn about disposable bioreactors at BTEC.
Process-Oriented Training in Single-Use Technology
by Gary Gilleskie
At the 2016 BioProcess Theater, Gary Gilleskie spoke about the design, delivery, and outcomes of a single-use processing short course offered through BTEC’s professional development program. The hands-on course covers single-use options from vial thaw (working cell bank) to vial fill (drug product) and is delivered in collaboration with single-use suppliers. The course serves as an example of the unique professional training opportunities that an educational center such as BTEC can offer in response to the adoption of new technologies.
The single-use course that is the subject of this article is part of BTEC’s open-enrollment short course program, which currently offers 16 different courses annually. We frequently seek to identify gaps in topics covered and in 2009 first considered the need for a short course on single-use technologies for biopharmaceutical production. Adoption of single-use technologies has been rapid since use of disposable bags for solution storage first began in the late 1990s. In fact, recent surveys show that since that time, more than 80% of biopharmaceutical manufacturers use single-use systems (1). Further, very few courses on the topic existed in 2009. Rapid adoption of the technology combined with a lack of training and education opportunities strongly suggested a need for a short course for professionals on the topic of single-use technologies.
BTEC’s first short course on single-use technology was held in 2010. It was a lecture-only course delivered over one day — significantly different from our current course. Multiple offerings of that course were attended by more than 50 professionals. Participant feedback obtained through postcourse evaluations highlighted the need for more comprehensive coverage of the material. It also revealed that a single day was not enough time to cover the breadth and depth of topics that interested participants. For example, they wanted details on options for all upstream and downstream biopharmaceutical processing steps, all of which is a challenge in a one-day course. Participants were also interested in hands-on laboratory activities in addition to lecture. And trainees wanted material that covered up-to-the-minute trends, a challenge in the evolving single-use landscape. The feedback from our first attempt at a single-use course led to a significantly refurbished course first held in 2015: “Hands-On Single-Use Processing for Biopharmaceuticals.”
Design and Development: Feedback from the previous version of the single-use course informed the design of the refurbished course. To meet the request for hands-on experiences with single-use components and equipment, the refurbished course includes laboratory experiences in addition to lecture. Further, laboratory activities are limited to 12 people to ensure that the training is, in fact, hands on.
To address feedback calling for more comprehensive coverage of single-use technology, the course was expanded from one to three days. And a broad set of learning objectives was written. These objectives, shown below, complete the statement “By the end of the course, participants will be able to . . . ”
Describe the latest single-use options from vial (working cell bank thaw) to vial (drug product fill)
List the advantages and disadvantages of single-use technologies relative to multiuse equipment
Identify process scenarios in which single-use technologies offer advantages over traditional multiuse equipment and vice versa
Set up and operate single-use systems for both upstream and downstream processing
Perform basic cost calculations to compare single-use options to multiuse equipment
List disposal options for single-use components and speak to the environmental impact of single-use and multiuse processing
Describe expectations for validation of single-use systems, including extractables and leachables testing.
The course was designed for a broad audience, including process development scientists, process engineers, manufacturing managers and supervisors, validation and quality assurance professionals, and suppliers of single-use components. Recognizing that such an audience would have different levels of process experience/knowledge, we included coverage of the underlying principles of the various unit operations used in biopharmaceutical processing. So a participant who may have expertise in chromatography but know little about bioreactors, for example, would receive basic information on cell growth and basic bioreactor design before covering single-use topics related to cell culture. This ensures that single-use topics can be understood in the context of basic process considerations.
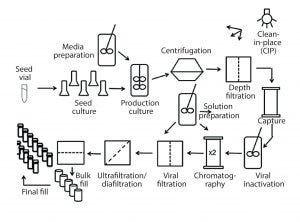
Figure 1: Process flow diagram for monoclonal antibody production using CHO cells; this figure was used to guide coverage of different unit operations and ensure that course material was in the context of a complete process for biopharmaceutical production.
Unit operations were presented in the order in which they are typically used for biopharmaceutical production. The process-flow diagram shown in Figure 1, which represents a typical Chinese hamster ovary (CHO)–based process for production of monoclonal antibodies, was used to guide course topics. This ensures that all unit operations are covered — including basic principles and single-use options — and are presented within the context of a complete process for biopharmaceutical production. Stated in another way, this course is not about how single-use technologies apply to only one processing step. Nor is it focused solely on the setup and operation of one particular operation. The coverage is broader and deeper than that, and operations are presented in the context of a complete biopharmaceutical process.
In addition to providing discussions of each individual unit operation, the course covers a number of other topics critical to implementation of single-use technologies. These include cost comparisons between single-use and multiuse options, leachables and extractables considerations, and environmental impact of single-use processing. Expanding the course to three days allows for comprehensive coverage, as participants in the previous version of the course had requested.
Implementation: Given that one primary objective is to provide participants with hands-on experiences in most unit operations comprising a typical biopharmaceutical process, appropriate equipment is necessary. BTEC’s laboratories are equipped with many single-use systems (Photo 1). However, we did not have single-use equipment for every unit operation. To ensure that most unit operations are covered in the laboratory and to ensure that students are exposed to the most up-to-date commercially available options, we reached out to suppliers for support. Pall Corporation stepped up and worked with BTEC to fill gaps. For example, Pall lent an Allegro MVP system with a Stax chassis for a depth filtration lab, and it lent an Allegro single-use tangential-flow filtration system for an ultrafiltration/diafiltration laboratory.
Pall also lent subject-matter expertise to help run laboratories and to provide lectures on certain topics in which expertise was not available internally.
The first offering of the “Hands-On Single-Use Processing for Biopharmaceuticals” course was held in October of 2015.
Evaluation: We had 10 participants in total for the 2015 first offering, just two shy of capacity. Three were from academia, and the other seven were from the biopharmaceutical industry or companies that are single-use suppliers. Participants’ biomanufacturing experience ranged from less than two to 16+ years.
A postcourse evaluation asked a number of questions. Two of the most important were “Did the course provide the information and skills necessary to effectively carry out my job?” and “Now that I’ve completed the course I have a greater understanding regarding the use of single-use technologies(?)” All participants agreed with both of the questions, most showing strong agreement. Additionally, written comments from the students were very positive. Noted strengths included seeing the entire process from cell culture to final fill, the comprehensive nature of the course, and availability of the most up-to-date single-use options. So we were able to improve on many weakness noted in the first version of the single-use course.
The Future of the Course
BTEC has put together a well-received, comprehensive, process-oriented course on single-use technology. This course includes both lecture and hands-on laboratory experiences and covers most unit operations required for biopharmaceutical production. Postcourse evaluations show high satisfaction with the initial offering. This type of course serves as an example of the unique professional training opportunities that educational centers such as BTEC can offer, particularly in response to growing application of new technologies such as single-use equipment and components. Training centers and other academic institutions have the ability to assemble the variety of resources — both subject-matter experts and equipment — required to offer a hands-on course of this breadth. In fact, since BTEC first delivered a single-use course in 2010, a number of good single-use training options have become available from suppliers, universities, community colleges, and other training providers.
Critical to the success of BTEC’s course was its collaboration with a major single-use supplier (Pall Corporation). This collaboration allowed us to include laboratories for most unit operations within a biopharmaceutical process and ensured that the latest commercially available technology was being used.
A second course was offered in October of 2016 to a full classroom. Recognizing the value that single-use suppliers bring to the course, the 2016 offering brought additional support from Sartorius as well as Pall. Labs included new single-use options for upstream processing like the Sartorius Biostat STR-50 bioreactor as well as downstream technologies including the Cadence Acoustic Separator device from Pall for continuous removal of cells and cell debris from process streams and the Cadence BioSMB continuous chromatography system with disposable flow path.
BTEC will continue to offer the course into the foreseeable future and will continue to involve equipment/components from a variety of suppliers.
Reference
1 Peters RC. Technologies and Practices Must Evolve to Meet Demand. BioPharm Int. 28(1) 2015: 18–20.
Gary Gilleskie is the director of operations at the Biomanufacturing Training and Education Center (BTEC) at North Carolina State University, [email protected].

Photo 2: FDA students learn how to operate centrifuge controls for downstream operations.
Emerging Technologies: The Challenge of Automation Competency
By Scott Sommer
At the 2016 BioProcess Theater, Sommer explained the importance of improving the quality of and access to automation training and existing certification programs. In the pharmaceutical world, automation is going to affect many different aspects of your process. It will touch not only on manufacturing, but also on the collection of data, the creation of reports, product quality, and the way information is shared with management and the boardroom.
An Automation Competency Model has been developed in the past couple of years by the Automation Federation, a trade organization focused on emphasizing that automation is a profession worthy not only of support by industry, but also by the government (www.automationfederation.org).
The competency model details what an automation engineer should know. It starts with the basic points such as what you should learn in college, what you should learn just to be a professional, and things that you need to know as a business person. Then it describes what you need to know specific not only to automation, but also to an organization. The Association of Engineering Societies has a similar engineering competency model for mechanical, pharmaceutical, electrical, and the rest of the engineering disciplines. Both models provide good guidance about what your engineers should be capable of doing.
Currently, no one source lists competencies that you need in automation, and very few automation curricula exist in the United States. In fact, until recently, the US Department of Labor did not recognize automation as a profession. Therefore, when governors went to get monies for their colleges and universities, automation was not on the list of professions that could obtain funding from the Department of Education. There is no way to read and study “into competency.”
There is no “read-and-understand” way to become truly competent in your field. The same is true for automation. But companies have limited training funds, and those can be spread pretty thin. What you get trained on today can become obsolete tomorrow or in a year or two. Company needs also change and expand, so training in automation must be able to do the same. And companies have differing focus areas. Many requirements overall relate to quality, cost, throughput, efficiency, compliance, and customer satisfaction. Automation engineers deal with a small aspect of an overall process focused on performance, device integration, technology, data, and networks (Photo 2).
In previous years, we hired for competency. We may have needed someone who knows the DeltaV system (Emerson Process Management), or who knows a batch platform, or someone who knows the historian that company uses. But such an approach fails to develop current staff and results in silos of expertise within a company. Also, a company will focus primarily on training in compliance and standard operation procedures (SOPs) because those are the largest immediate areas of concern, especially when products need to remain compliant with regulations. However, successful training encompasses many other aspects. So you need a roadmap to get from where you are to where you need to be.
My suggestions for developing a training model for automation engineers fall into the seven points below.
Realize That Training Is a Process: We need to think of automation development and training as a process, something that needs to be planned. It can’t be done haphazardly or dictated by a company because trainers don’t know where everyone is starting and what each employee wants in terms of professional development.
Learn the Basics: Engineers who know programmable logic controller (PLC) programming very well might not understand that before you start a pump, you need to open the upstream valve to let fluid into that pump. They understand the programming and the mechanics, but they don’t have the basics of fluid mechanics and other basic process knowledge.
Know That Formal Training Is Only the Beginning: You can’t go to college and come out trained in your profession. I have always abided by the mantra from one of my professors: “College makes you trainable. When you start your first job, you won’t know how to do anything, but you’re ready to be trained to do whatever that is.”
Develop Personal Initiatives: Successful training requires the initiative of automation engineers or technicians themselves. They need to have the wherewithal and the desire to learn and expand their horizons.
Assess Goals and Milestones: If you listen to CEOs talk about how they came up through the ranks, they stress that they had goals and milestones. They assessed whether they were achieving those and if not, why not?
Benefit from External Sources of Expertise: We need to leverage vendors and professional societies so we can interact with like-minded professionals. That’s the biggest, untapped source of free knowledge there is. I learn more from the vendors and professional societies than I do from going to any course or attending any webinar. I enjoy leveraging such contacts because they are the experts in their particular field.
Recognize That Learning Never Stops: The most important thing to realize is that this is a lifelong learning process. I’ve been in the industry for over 35 years, and I learn something new every day. When I do, I am sure to comment on it to my team. “Hey, I didn’t know that before.” This helps reinforce within my work group that learning never stops.
Planning for Your Own Professional Development
Developing a training program is a process. Building on the points listed above, here are some further suggestions for planning for your own professional development.
Make a Plan, Conduct a Skills Inventory: The Engineering Competency Model has a checklist (a gap analysis) that you can use to figure out what you know and what you want to know. Know your position requirements. Do you need to know something about databases? Is SQL something that you need to know to get data out of them? Do you really fully understand what your job is and how to identify where you need additional training?
Plan for Technical Mastery: Don’t just read about things. Look for mastery. The only way to master something is to actually do it. Ask to be put on a project that requires a particular skill that you just learned.
Develop a Training Matrix: This is the first thing you need to do to develop your goals. And define a method of validating or verifying your own competency. For me, it was passing the Certified Automation Professional Test because it tested all of the areas in the competency model and the task skills analysis. I knew that if I passed that test, I had the skills, the knowledge, and the prerequisites to call myself an automation professional.
Look at Standards and Best Practices: Find out what those standards and best practices are for you (see the “Organizations and Certifications” box). For instance, for pharmaceutical projects, it’s ISPE‘s good automated manufacturing practices (GAMP) that you want. Go after your CPIP certification. I’ve earned both a CAP and CCST certification. Those were goals I set for myself. Learn what PMI has to offer if you’re in project management or you’re a technical lead.
Organizations and Their Certifications Mentioned in This Article
Automation Federation (www.AutomationFederation.org) Publication:Automation Competency Model |
|---|
BICSI Building Industry Consulting Service International (www.BICSI.org) Certifications: RCDD (registered cabling and communications designer) |
ISA International Society of Automation (www.ISA.org) Certifications: CCST (certified control system technician); CAP (certified automation professional) |
ISPE International Society for Pharmaceutical Engineering (www.ISPE.org) Publication:GAMP (good automated manufacturing practices) Certifications: CPIP (certified pharmaceutical industry professional) |
PMI Project Management Institute (www.PMI.org) Certifications: PMP (project management professional) |
Look at the Technologies from a Standards Perspective: Maybe your plant uses Foundation Fieldbus but not other bus technologies. Learn them anyway — they are all pretty much identical. Many of them were based on exactly the same technology, but because of infighting among societies, they branched off and formed their own programs. But it’s good to know what those are because each one of those technologies has a specific place in industry where it is the best method for a particular task.
Look at Programming, Device Connectivity, Data Integration, and Hardware Integration: There is a lot to know. Find out what you do know, what you’d like to know, and then what you need to know, and seek out training to fill those gaps.
Where Do You Get Training? You can get formal training. Go to a class. Enroll in a degree program. Professional societies and consultants have webinars or preprogrammed training programs or even white papers that give you different perspectives. As an employee, you need to help train yourself. Read. Ask questions. Interact with people in the field.
How Are You Going to Get the Training? Programs include instructor (classroom-led), web-based, multimodal, self-paced study, experiential, and peer-to-peer formats. Don’t just lock yourself into, “Well, I can’t leave work, so all I can do is what they give me in-house” or “You know, I really like to eat my lunch, so I don’t want to participate in lunch-and-learns because that’s my time to go sit under a tree.”
Take Initiative. Keep a Journal: Use the gap analysis at www.automationfederation.org or www.aaes.org. Look at those competency models, and print out the spreadsheets. You will read something such as “Be able to solve a calculus problem using . . . .” Then ask yourself, Do I know how to do that? Yes or no? Do I know what a 4-to-20 milliamp loop is? Can I explain it to somebody else? Yes or no? Go through that analysis, and find out what it is that you need to know to perform your job better and then to improve your technical expertise.
Spend Some Money Yourself: Don’t depend on everybody else. From time to time I see a course I’ll spend $500 for. Sometimes it was a good investment and sometimes not so good. But I was able to achieve what I call some knowledge capital: some knowledge that I can use later to profit from what I like to do. Most automation engineers have chosen the profession because they love to do it.
Set Goals and Milestones: You are going to become licensed. You want to get a PE or a CAP or an RCCD (for a registered cabling and communications designer, a person who assembles big data networks). Maybe you want your project management professional designation. Maybe you want to get into a leadership position. What does it take to do that? Tell someone in your company: “I want to become a project manager or an engineering manager or a project lead.” Set your goals and achieve them.
Share Your Goals with Your Supervisor, and Use Achievements for Additional Opportunities: Sometimes an opportunity just pops up. Sometimes you have to search for it and say “I’ve got this training or this knowledge. I’d like to work on this project.” Or sometimes it just falls into your lap and somebody says, “I can’t find anyone with this experience.” Raise your hand, and voila.
Leverage Other Professionals. Find a Mentor: If you don’t have a mentor, find one. I’m 57 and I have a mentor — someone who knows a lot more than I do. If you are just starting out in the industry, everyone knows more than you do. But be selective. Find someone who knows what it is that you want to learn. When you get to the point of actually having expertise in an area, become a mentor. Make sure that if you are a supervisor, you have people under you that you mentor. Don’t just supervise them, mentor them. Help them to grow. My goal is that all those who work for me should be able to do my job at some point in their careers.
Use Vendor Support and Sales Groups for Basic Training: The only way I learned how a pump worked is that a Durco Pump salesman came to the plant where I was working. We disassembled a pump and put it back together. Before then a seal to me was one of two things: a food-preservation container or an animal. Vendors are an excellent source of practical information about how things work.
Look for Professionals in Adjacencies: I don’t really work in the pharmaceutical arena anymore, but in an adjacency, a bio-refining role. We use many of the same instruments and processes that are used in pharmaceutical companies. I was able to transport what I knew from the pharmaceutical world into my role at Renmatix.
Don’t Be Afraid to Ask Questions: Admit that you don’t know something because that’s the first step in actually getting to know it.
A Lifelong Process
Learning is lifelong. You’ll never know everything. Unless you keep on striving and learning, technology is going to leave you behind. Everything learned will make a difference later. Everything you learn now you’re going to keep, especially if you have hands-on experience. That’s going to benefit you later. Learning over time is exponential, so take advantage of it. Treat learning as a journey with no end.
Automation competency is realized through training and development, which is self-actuating: It starts with you, it is role-driven, and it is ongoing. You need the support of your company, but you also must take initiative to seek out the training that you need to meet your personal and professional goals.
Scott Sommer is a technical fellow, automation and controls, at Renmatix; [email protected].
You May Also Like






