The
BPI West conference
is always a “candy store” of timely presentations and key networking opportunities for an editor who cannot be in all places at once. This year’s event in San Francisco (27 February – 2 March) was no exception. Nearly 900 delegates were joined by 86 exhibitors and 48 poster presentations. I’m inviting a number of speakers to convert their talks into manuscripts for BPI.
In focusing on late-stage processes and commercial trends at this year’s event, I discovered a partnering aspect to most tracks and presentations. Collaborations are increasing overall between suppliers and end users. Given the mantra of faster, more efficient, and cost-effective development and biomanufacturing, speakers emphasized a need to find points of commonality and streamline efforts at all stages.
Progress in
regenerative medicine
is highlighting ongoing logistical challenges and the need for automation. As one speaker noted, a manufacturer of autologous therapies could find itself managing up to 2,500 ...
WHO's deadliest bacteria list; ESCMID and ASM explore antimicrobial resistance; Ebola vaccine protests; Antimicrobial peptides as a substitute for antibiotics
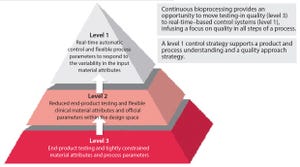
Figure 1: A guided decision process for continuous bioprocessing (
1
)
The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits.
Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it is difficult to displace existing processes. But for early stage pipeline biologics, adopting new process technologies can be easier from a regulatory and a manufacturing strategy perspective. The biopharmaceutical industry has not yet fully embraced CBP models. However, it is maturing and expanding globally, which means its willingness to adapt to meet different needs and requirements is ch...
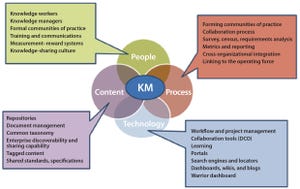
Figure 1: Process technologies — key themes
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only through process technologies available to the industry, but also through enhancements to the way in which those technologies are implemented. Automation, analytics, modularity, and knowledge management all have key roles to play in this transformation. In part 3 of this series, we discuss how those factors will shape the industry’s future.
Thirty-one member companies contributed to t...

 +2
+2WWW.PHOTOS.COM
Exposure to solid–liquid and air–water interfaces during production, freezing and thawing, shipment and storage of protein therapeutics may be a contributing factor in their degradation (e.g., aggregation, fragmentation) (
1
,
2
). Surface exposure, particularly during manufacturing processes, often is accompanied by various degrees and durations of shear stresses originating from fluid flow and acting on proteins at interfaces. The magnitude and duration of shear rates depends on velocity gradients within each solution and varies significantly among manufacturing steps. On the low end, a shear rate of 50 s
–1
(inverse seconds or Hertz) is applied to biotherapeutics during mixing processes typically lasting from minutes to hours. On the high end of the range, shear rates of up to tens of thousands of Hertz arise during filling (up to a million during high-pressure homogenization), but those usually are limited in duration to mere seconds (
3
). Additionally, proteins may be subjected to h...

 +6
+6WWW.GRAPHICSTOCK.COM
Nadine Ritter is president and senior analytical advisor of Global Biotech Experts, LLC and a long-time member of
BioProcess International’s
editorial advisory board. At a recent CASSS North American CMC Strategy Forum called “Methods on the Move: Addressing Method Transfer Challenges,” she discussed the biopharmaceutical industry’s logistical challenges of analytical test samples for drug substances and products. At the conference, BPI’s editor in chief Anne Montgomery met with her to discuss some key points of this topic.
Logistics Challenges
Montgomery:
What are some main issues for contract laboratories regarding sample testing?
Ritter:
One issue for analytical testing programs (contract and in-house) is that samples needing analysis must be transferred from where they’re taken (e.g., from a manufacturing floor or stability chambers) to testing locations. For in-process testing, this usually is not so much of a problem because those laboratories tend to be close enough to man...

 +1
+1Photo 1: Clones exhibit a number of behaviors, one of which is clumping.
Biotherapeutic proteins usually are produced by either fed-batch or perfusion processes. Perfusion manufacturing can provide much higher levels of productivity than fed-batch systems can, thereby reducing production costs. A 2013 study showed that perfusion is more cost effective than fed-batch processes for most combinations of titers and production volumes (
1
). Moreover, because a perfusion process is much closer to steady state than is a fed-batch process, it often produces a more consistent product — especially for molecules that are sensitive to changes in conditions inside a bioreactor.
That said, perfusion is not used as often as it could be for producing biologics, largely because it is more complex to set up than fed-batch processes. Perfusion requires the continuous removal and replacement of cell-culture fluid with fresh media, while maintaining a constant volume in a bioreactor. Additional complexities can come from pos...
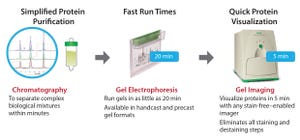
Figure 1: Stain-free gels are more sensitive than Coomassie-stained gels. TGX stain-free gels (BioRad Laboratories) were loaded with standards and a serial dilution of a protein mixture with four different proteins of varying tryptophan (Trp) content: phosphorylase B (PB, 1.4% Trp, 97 kD), glutamic dehydrogenase (GD, 0.8% Trp, 56 kD), carbonic anhydrase (CA, 2.3% Trp, 31 kD), and lysozyme (Lys, 3.4% Trp, 14 kD). Gels were run for 20 min at 300 V and imaged using a stain-free enabled imager (left). Gels were then stained with Coomassie stain and imaged using the same imager (right).
Purification of recombinant proteins is a critical step during protein therapeutics development. Protein therapeutics have a number of classifications based on their potential applications, including use as vaccines and diagnostics as well as for enzymatic, regulatory, or targeting activities (
1
). For all such applications, identifying and verifying protein purity is most important. Whether proteins themselves are therapeutic...
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (
1
,
2
). In addition to cost savings, as Ernst & Young authors wrote in a February 2015 BPI special report, “Genetically engineered vaccines hold the promise of increasing safety, reducing reactogenicity, and improving immunogenicity” (
1
).
Universal Approach Shows Promise
An international team of scientists have designed a new generation of “universal” influenza vaccines that could help protect against most (88%) known flu strains worldwide (95% of known US strains) with...