CellPrime™ Recombinant Human Transferrin As an Animal-Free Alternative to Animal-Derived Transferrin or Iron Supplementation in Cell Culture ApplicationsCellPrime™ Recombinant Human Transferrin As an Animal-Free Alternative to Animal-Derived Transferrin or Iron Supplementation in Cell Culture Applications

Figure 1.
CellPrime rTransferrin recombinant human transferrin provides an animal-free alternative to iron salts for industrial cell culture. It is a recombinant analogue of human transferrin expressed in Saccharomyces cerevisiae. Supplied as a human holo-transferrin analogue, CellPrime rTransferrin binds specifically to the transferrin receptor, thereby facilitating iron uptake into cells for optimal cell culture performance. Here we describe cell culture data demonstrating that CellPrime rTransferrin shows equivalence to human transferrin (hTf) and superiority to bovine transferrin (bTf) in stimulating cell growth and protein production across a number of industrially relevant cell lines.
Results
Human Recombinant and Native Transferrins Promote Greater Growth and Protein Production than Bovine Transferrin: To compare the efficacy of CellPrime rTransferrin to commonly used transferrin media supplements, it was compared with serum-derived human transferrin (hTf) and bovine transferrin (bTf) at identical concentrations (5 µg/mL) in the CHO DP-12 cell line. At days 6 and 10 in culture, CellPrime rTransferrin promoted similar increases in growth (Figure 1A) and protein production (Figure 1B) as native hTf. By contrast, bTf showed little increase in cell growth over SFM, with significantly greater effects observed at day 10 than at day 6. Similar results were observed at day 10 in culture (data not shown). These data suggest that CHO cells respond preferentially to human transferrin in either native or recombinant form.
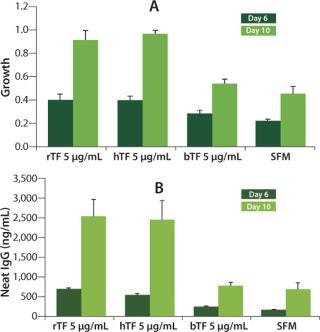
Figure 1. ()
CellPrime rTransferrin is More Effective than Iron Salts at Promoting Growth and Protein Production: Iron-salt supplementation is another commonly used method for enhancing CHO cell performance. In this experiment, iron salts were added to a commonly used off-the-shelf medium (DMEM/F12) that already contains a nominal amount of iron. For these experiments, CellPrime rTransferrin (5 µg/mL) was tested against iron salts (1.3 µM) using the same experimental model as described above in CHO DP-12 (Figure 2a and 2b) and CHO B13-24 (data not shown) cells. The results indicate that all treatments with CellPrime rTransferrin performed equal to or better than those with iron salts for both growth and protein production in each CHO clone. The increased growth and protein production were again more pronounced in the DP-12 cells than in the B13-24 cells, as observed in experiments described above. These data indicate that CellPrime rTransferrin’s benefit is derived from its biological activity as an iron carrier rather than from the addition of iron bound to it. The amount of iron added with the CellPrime rTransferrin is much lower than that added to the medium by adding iron salts.
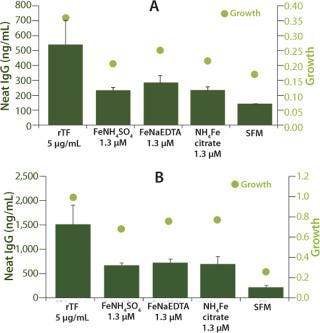
Figure 2. ()
Discussion
The introduction of CellPrime rTransferrin provides an animal-free alternative to iron-salt supplementation that permits biological (not chemical) iron supplementation in cell culture media. This is an important distinction: Data comparing the effect of CellPrime rTransferrin to iron salts suggest that biological delivery of iron promotes growth and protein production to a degree not seen with iron salts alone. Here we also demonstrate that CellPrime rTransferrin supports cell growth and protein production equivalent to hTf (and superior to bTf), indicating that supplement species choice can substantially affect cell culture performance as well. The low concentration of CellPrime rTransferrin required to achieve increased cell performance makes this a cost-effective option for providing optimal iron supplementation to cells.
In summary, CellPrime rTransferrin provides a superior alternative to commonly used transferrin or iron supplements in cell culture by eliminating the use of animal-derived components while enhancing cell performance.
Additional experiments are planned in customer-relevant assays at larger scales (e.g., shake flasks and bioreactors) with cells grown in completely serum-free conditions to verify the findings of this study.
You May Also Like






