A Perspective on GMPs for Cellular Therapy CommercializationA Perspective on GMPs for Cellular Therapy Commercialization
April 17, 2019
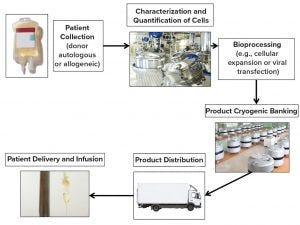
Figure 1: Lifecycle of cellular therapies
Cellular therapies can be classified by therapeutic indication, by cell types, and by whether cells are taken from and administered to the same individual (autologous) or derived from healthy donors (allogeneic). Regulatory classification of cellular therapies differentiates among minimally manipulated cells for homologous use, transplants or transfusions, and cells that are more than minimally manipulated and regulated as medicines. Medical cellular therapies must meet quality, safety, and efficacy standards to obtain marketing authorization (1–8). Such therapies can be subdivided into somatic cell, gene therapy, and tissue-engineered products. They can be manufactured from autologous or allogeneic sources and contain noncellular components (e.g., chemical or biological compounds and matrices).
Off-the-shelf allogeneic products are based on cells obtained from healthy donors and modified with gene editing to knock out functions such as immune rejection responses. The modified cells are expanded to produce large quantities of product that can be frozen and stored until needed for patients. Patient-specific cell therapies are most often autologous, but they also can be allogeneic from matched donors to prevent immune rejection. The key difference between patient-specific cell therapies and off-the-shelf products is that the former requires a unique manufacturing batch for each patient. Gene editing has proven to be successful for commercial patient-specific cell therapies such as for adoptive cell transfer with chimeric antigen receptor T (CAR-T) cells (Figure 1).
Regulatory Requirements
Regulations already are in place for minimally manipulated cellular therapy products. These regulations focus on preventing the introduction, transmission, and spread of communicable diseases. One requirement covers establishment registrations, donor eligibility standards, and current good tissue practices (CGTPs) for processing cellular products (1–8). These products are regulated as drugs, medical devices, and biological products, which adds the regulatory requirement of manufacturing under current good manufacturing practice (CGMP) conditions (9–11). As cellular therapies move from clinical to commercial manufacturing, regulatory agencies evolve regulations using a risk-based approach. Regulations are being established that will permit the manufacturing of safe and effective cellular therapy products. Scientific and technical developments still are needed to help cell therapy manufacturers apply processes for CGMP manufacturing and quality control (QC).
Cell Source
Source-cell variability must be addressed to ensure product consistency. Some manufacturers of patient-specific products provide both detailed training and specialized collection kits to address such variability. Because source-cell materials have such high values, collecting or producing ample amounts for process development and validation is an industry challenge. Limited shelf life and quantities of cells can complicate QC testing and stability determinations.
Banked cells must be characterized extensively for potential contamination with microbial or viral agents as well as for tumorigenicity. Determining critical quality attributes (CQAs) for products and developing assays to monitor their potency are essential to the commercialization of cellular therapy products. Characterization of both source cells and final cellular products is important for autologous and allogeneic therapies. Source cells are characterized based on the presence of surface markers, cell size, and combinations of attributes associated with cell source and mode of action. Optimal characterization is essential to increasing the accuracy of cellular specific population selection for producing cell therapies.
Autologous or Allogeneic
Autologous and allogeneic products exhibit different features and present different manufacturing challenges. Autologous product challenges include patient variability and the need for small-scale manufacturing lots. They can provide immunogenicity benefits because of their patient-specific nature. Allogeneic products are considered to be less complex because they often use cell banks that can be characterized for safety and scaled up to make mass-produced universal products for a large number of recipients. But complications for such products include potential immunogenicity (either from a recipient, in which case cells survive only for a short time after administration, or in Graft-versus-host reactions), potential tumor formation, and the requirement of appropriate manufacturing platforms.
For autologous cellular therapies, a patient is both the raw-material source and the recipient. So the focus is on the collection, processing, and readministration of cells from and to an individual patient. Autologous therapies are advantageous for patient safety because they eliminate concern about Graft-versus-host disease. For these therapies, a closed manufacturing system should be used to reduce risk of product contamination. Individualized batch records for processing documentation should be accessible at the collection point. Materials should be traced throughout an entire lifecycle, including during shipment, manufacturing, and patient administration. Because cell therapies are time sensitive, it may be feasible for healthcare professionals to process cells using automated systems at the site of collection. That process could include cell collection, characterization, processing (e.g., with viral transfection), and readministration.
For allogeneic cell therapies, cells from one donor serve as the source material for treatments for multiple patients. Donation logistics can be complex if the number of cell expansions is limited, so additional collections from the same donor may be required. Allogeneic product manufacturing is more feasible for automated cell processing because all cells in a batch come from a universal donor. Scaling up production from development scale to manufacturing scale can be challenging because it involves different processing systems for cell expansion, volume reduction, and harvest. Manufacturing equipment used to scale up manufacturing such as single-use bioreactors with microcarriers cannot yield the same product potency as flasks and trays used for research and development.
Collection and Processing
Raw materials and components used in manufacturing cell therapies must be evaluated carefully. These include media components, matrices for cell attachment, culture vessels, tubing sets, bags, and other disposables. Testing extractables and leachables is required for single-use components such as culture vessels, tubing sets, and bags because such materials can compromise cell growth and viability as well as patient safety. Recombinant serum proteins should be used instead of animal- or human-derived sera to prevent the risk of bacterial and viral contaminations.
Vendor qualification procedures should be well established: through vendor screening, a site audit, a quality agreement, and vendor monitoring. Each new lot of component materials should be tested, and biomanufacturers should have the accompanying certificates of analysis (CoAs) generated by vendors to ensure that such components meet specifications.
Healthcare professionals who perform collection procedures typically are not trained in CGMP regulations. Collection documentation should become part of CGMP records, including electronic data generated from collection equipment and on-site testing. Cell manufacturing locations depend on the types of cell products and the application of those products. The decision between a bedside point-of-care or a centralized manufacturing model should be based on product indication and product stability.
Cell therapy manufacturing performed off-site from collection locations requires strict control in CGMP facilities. This includes all manufacturing spaces, storage warehouses for raw and finished products, and laboratory areas (12). Cellular therapy manufacturing facilities must be designed for aseptic processing. Development of fully enclosed manufacturing equipment and built-in controls for in-process testing is optimal for processing. Process flow must be considered from clinical to commercial manufacturing using a quality by design (QbD) model to validate CGMP manufacturing processes. That involves defining critical process parameters (CPPs), process parameters, and in-process tests. Such information should originate from development studies and be used to define a design space for process validation. Those parameters and tests should be monitored continuously as part of on-going process validation. Manufacturers that do so may enjoy regulatory flexibility to operate in defined design spaces as well as reduced testing because quality is built into their manufacturing process designs and documented as such.
Other important validation and verification activities include qualifying manufacturing facilities and equipment, developing facility and equipment cleaning validations, developing analytical testing and method validations, developing environmental monitoring programs, developing batch records, and developing an on-going process validation plan. These validation and verification CGMP requirements should be met by incorporating data integrity best practices (both in manufacturing and in laboratory testing) using attributable, legible, contemporaneous, original, accurate (ALCOA) documentation, sterile processing best practices, and chain of custody best practices (Editor’s note: For more information about data integrity, see this month’s featured report).
Manufacturers must put procedures in place to prevent microbiological and cross-product contamination attributable to raw materials, environmental conditions, and handlers. They must establish robust methods for container sterilization and control of raw materials and reagents. Use of high-efficiency particulate-absorbing filters and cleanrooms can prevent airborne cross-contamination of materials from environmental contaminants or other products. Parameters such as temperature, humidity, and pressure should be monitored because of their relationships to particle generation and microorganism proliferation.
Materials and staff flows should be separated and be unidirectional to minimize cross-contamination. Operators should be trained in aseptic
processing techniques for cleanrooms (13).
Feedback automation processing is optimal for cell therapy manufacturing. Variability of source-cell properties is addressed best with manufacturing processes that are flexible and adaptable to ensure that end products are of same high-quality and consistency. Feedback can control culture conditions in-process based on real-time measurements of CQAs (14). Integration of in-line assays and measurements for CQAs as well as rapid measurements of potency, efficacy, and safety parameters are essential elements of automating cell manufacturing processes and will enable establishment of robust CQAs.
Each product batch should pass specific tests unique to that product’s characteristics. Tumorigenicity and biocompatibility testing should be performed where appropriate (15, 16). Extensive product characterization is essential for process validation and development of in-process and release-testing specifications.
Cell therapy manufacturing does not allow for terminal sterilization of final products or removal of microbial contaminants with filtration. So it is important to test starting materials and validate aseptic manufacturing processes to ensure that products are free of contamination. Sterility testing for the absence of bacteria, mycoplasma, and fungi should be conducted at the time of product release whenever possible. Some cell therapy products require release and administration to patients before all required compendial testing can be completed. In such cases, rapid test methods can be used. For example, mycoplasma detection based on polymerase chain reaction (PCR) and rapid microbial detection methods have been used in release testing of cell therapies because such methods enable early read-outs on potential microbial contamination. A 14-day sterility test still must be completed even after patient administration.
Living cells are dynamic. They can continue to grow, differentiate, migrate, and interact within a human body. Characterization of cell populations before administration does not describe fully the phenotypes and genotypes of cells in patients after cellular treatment. Characterization and CQAs should be redefined based on feedback from patient data and improved understanding of mechanisms of action as patients are monitored following treatment. Using posttreatment patient data to understand the most effective therapeutic cells can decrease the number of cells required for treatment and manufacturing timelines.
Distribution Procedures
Most cell therapies do not remain viable at ambient temperature over extended periods. This applies to both allogeneic and autologous cell therapies. Autologous cellular therapies have additional challenges when a patient’s cells must be transported to a processing facility and back for patient treatment.
Allogeneic products that are stable enough to be shipped globally could be supplied from a single central manufacturing site, thus providing efficiency and consistency of production. However, products with limited stability (both autologous and allogeneic) might require a distributed model in which manufacturing occurs in multiple regional centers or are even at sites of treatment. Shipment of cellular therapy products must be validated and temperature monitored. Chain-of-custody and cellular environmental-condition records should continue from collection through to administration.
Potential of Cell Therapies
The use of cellular therapies can move beyond conventional disease treatment by addressing underlying causes of disease, altering its course, and reversing damage that already has occurred. Transitions from discovery through research and development to commercially manufactured products require biomanufacturers to incorporate CGMP regulations into the collection, production, and delivery of these products. CGMP regulations for this new class of regenerative medicine will be defined by a collaboration of clinical, industrial, and regulatory agencies. Such developments will allow for cellular therapy treatments to become increasingly available to patients and will offer new treatments (and perhaps cures) for many diseases.
References
1 42 US Code §262: Regulation of Biological Products, 2010. US Government Publishing Office: Washington, DC.
2 EudraLex: The Rules Governing Medicinal Products in the European Union Volume 4 — EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Annex 2, Manufacture of Biological Active Substances and Medicinal Products for Human Use. European Medicines Agency: London, UK, 2012.
3 Guide to the Quality and Safety of Tissues and Cells for Human Application. European Directorate for the Quality of Medicines: Strasbourg, France, 2017.
4 21 CFR Part 1271: Human Cells, Tissues, and Cellular and Tissue-Based Products. Office of the Federal Register, US Government Publishing Office: Washington, DC.
5 21 CFR Part 600: Biological Products. Office of the Federal Register, US Government Publishing Office: Washington, DC.
6 21 CFR Part 200: General Drug. Office of the Federal Register, US Government Publishing Office: Washington, DC.
7 Regulation (EC) No 1394/2007: European Parliament and of the Council of 13 November 2007 on Advanced Therapy Medicinal Products and Amending Directive 2001/83/EC and Regulation (EC) No 726/2004. European Commission: Brussels, Belgium, 2007.
8 Guidance for Industry: Human Somatic Cell Therapy and Gene Therapy. US Food and Drug Administration: Rockville, MD, March 1998.
9 21 US Code Chapter 9: Federal Food, Drug, and Cosmetic Act, Section 351. US Government Publishing Office: Washington, DC.
10 Common Standards for Cellular Therapies. Foundation for the Accreditation of Cellular Therapy: Omaha, Nebraska, March 2015.
11 Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 (Human Tissue and Cells Standards). European Commission: Brussels, Belgium, 2004.
12 WHO Technical Report Series No. 986: Good Manufacturing Practices for Pharmaceutical Products — Main Principles, Annex 2. World Health Organization: Geneva, Switzerland, 2014.
13 ICH Q8(R2): Pharmaceutical Development. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, August 2009.
14 Guidance for Industry: PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance. US Food and Drug Association: Rockville, MD, 2004.
15 European Pharmacopoeia (PhEur), 9th ed. European Directorate for the Quality of Medicines: Strasbourg, France 2018.
16 USP 36–NF 31. US Pharmacopoeial Convention: North Bethesda, MD, 2013.
Kimberley Buytaert-Hoefen, PhD, is principal consultant at PAREXEL International, 195 West Street, Waltham, MA 02451; 1-720-417-6091; [email protected]; www.PAREXEL.com.
You May Also Like






