Cell and Gene Therapy Data Management: Solutions to Address Complex ChallengesCell and Gene Therapy Data Management: Solutions to Address Complex Challenges

HTTPS://STOCK.ADOBE.COM
At the Phacilitate Leaders World and World Stem Cell Summit 2019, held 22–25 January, Steve Goodman (head of drug product manufacturing at bluebird bio) and Robert Di Scipio (CEO of Skyland Analytics) shared the podium to address what the product and process data-management ecosystem looks like for cell and gene therapy (CGT) development and manufacturing. A starting point for their presentation was that CGT development presents significant data challenges: capturing and analyzing product development and manufacturing data, tracking the collection, transporting and maintaining the quality of apheresis material, and analyzing these data coherently with the pertinent patient medical records. Skyland and bluebird are collaborating to ensure that bluebird has a CFR Part 11 compliant data management system that supports data transparency and analytics throughout its supply chain — as well as enabling CPV (continued process verification) and APR (annual performance reports).
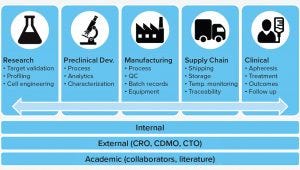
Figure 1: The cell and gene therapy data ecosystem (QC = quality control, CRO = contract research organizations, CDMO = contract development and manufacturing organizations, CTO = contract technology organizations).
Figure 1 provides a snapshot of data sources to be managed. Although these are not necessarily specific to CGT production, they show the complexity of data that are monitored throughout a product’s lifecycle. Also important to this field is that much of the work is performed in collaboration with research, development, manufacturing, and/or academic partners whose data are captured in multiple, proprietary data sources.
Early in product and process development, engineering and profiling data are generated to support process characterization and robustness, analytical method development, and validation of intended methods.
Process analytics and quality control (QC) testing is leveraged heavily in late-stage product development, and at this point, hundreds or even thousands of critical process and quality parameters may be captured in a batch record. In the case of autologous therapies, the desired data tracking can grow exponentially.
For CGTs, it is typical for external partners to handle the increasing complexity of shipping patient samples and collecting the related patient, chain-of-custody, and chain-of-identity data. Ultimately, a vast amount of information is generated in these stages that includes historical patient medical records and records from apheresis centers related to collections. Then data are generated by the treatment itself, the follow up, and management of the long-term registry. The rapid progression of assets to market, both in the stem cell area and in the T-cell space, can generate an overwhelming amount of data and associated complexities (see “Data Challenges” box) — especially when every patient’s data constitute a separate batch.
Data Challenges of CGT Supply Chains |
|---|
General |
Cell and Gene Therapies |
Regulations |
An additional level of complexity is the extensive reliance on contract development and manufacturing organizations (CDMOs or CMOs). Most drug companies typically rely on one or more third parties to assist in product formulation and process development as well as clinical and/or commercial manufacturing. Emerging companies may not have all the necessary expertise in-house, and global companies often are short on human resources and physical capacity. Therefore, most companies have a broad manufacturing supply network comprising internal teams working in conjunction with CDMOs and/or CMOs that generate batch records, quality release testing data, and certificates of analysis.
Goodman noted that the stage-specific categories of Figure 1 often can exist in isolation, increasing the challenges of generating links between them so supporting systems can connect to present data in aggregate. The obligation for long-term follow-up of patients to understand lasting impact is a tremendous responsibility that manufacturers need to address. Given the heavy reliance on CDMOs and the amount of variability in input material, apheresis, and output of a drug product, a product developer must navigate the complexities of linking product performance with incoming material.
In addition to supply-chain complexity, increasing scrutiny from regulators regarding data integrity is driving compliance expectations from an auditing perspective. If their expectations are not met, that can disrupt security of supply significantly.
Data Management By Design
Skyland’s CEO Robert Di Scipio noted that his introduction to the biotechnology space was through supply agreements he inherited at his biotechnology company that didn’t allow for the level of process and data visibility that regulators and good business practice required. He agreed with Goodman that more complex processes are generating substantially more data, especially in the regenerative medicine sector. CMOs and CDMOs recognize the need to share more data in real or “relevant” time, but they struggle with how to do that. A significant amount of data exists on paper, necessitating manual data transfer and segregation to share data with the right sponsor. If a digital system exists, those data sets still need to be parsed.
He also emphasized the increasing regulatory scrutiny, noting that 57% of the FDA 483 warning letters in 2018 were for data integrity, not for product quality. “The FDA knows that your approach to data management will have a direct impact on the quality of the drug product,” he said. The Securities and Exchange Commission (SEC) also is evaluating the sufficiency of investor disclosures made by public biopharmaceutical companies related to their reliance on external partners for product development and manufacturing. For example, is the level of data transparency sufficient? Can the product owners demonstrate sufficient process oversight?
Software As a Service (SaaS) Data Management: Cloud-based solutions to data management now constitute the technology of choice for IT departments. Initial reluctance of companies to adopt such solutions has given way to greater trust that such systems can be validated and share data securely. Because the FDA holds a sponsor liable for external manufacturing partners, a data management strategy should be in place concurrent with the establishment of an external development and supply network (see the “Compliant Virtual Manufacturing” box).
When embracing the need for SaaS data management solutions, developers/sponsors and external manufacturing partners of CGT products should begin by considering a comprehensive data solution that can be validated easily and that can work out-of-the-box without needing time-consuming and costly custom coding. Di Scipio recommends implementing a purpose-built solution early in product development that can be leveraged as a data library throughout the product lifecycle. He recommends that companies also seek out best practices for data management from industry peers and groups such as the BioPhorum Operations Group (BPOG). Di Scipio noted that the Association for Regenerative Medicine (ARM) also is working on supply chain best practices.
Another key requirement for data systems should be providing workflows and data management for CPV planning: the foundation for assurance that a process maintains a state of control. This requires integrating quality data and monitoring strategies, quality control tools, and data analysis methods.
Skyland has leveraged its experience in developing manufacturing informatics software for the biopharmaceutical industry to meet the industry’s compliance obligations and evolving supply chain challenges with a cloud approach. System capabilities include accelerating process control strategies and technology transfer by establishing a perpetual, collaborative workspace, monitoring target control limits, and managing product and process specifications. Pertinent experimental, manufacturing, and development documents and data can be attached or made available through internal or external links. The system contextualizes batch data with target control limits to generate autosegmented control charts. The system can generate a full audit trail of everything that’s been entered along with the rationale, authorship, and time stamp.
The program is designed to function as a data portal, with unit operations and process steps built into it through drop-down menus. It can be used by a drug sponsor to “onboard” to its CMOs. It enables CMOs to generate, record, analyze, and share data with customers. The system redefines technology transfer by providing a collaborative and secure workspace, allowing multiple sponsors and/or CMOs access to only that portion of data, product line, equipment train, or site that they are entitled to see.
Compliant Virtual Manufacturing |
|---|
Cloud-based solutions to data management (“data management by design”) should ensure |
Steve Goodman is head of drug product manufacturing at bluebird bio; bluebirdbio.com, [email protected]. Robert Di Scipio is CEO of Skyland Analytics; skylandanalytics.net, [email protected].
You May Also Like






