Characterization of Hydrogel Carriers: Improving the Efficacy of Existing Cell TherapiesCharacterization of Hydrogel Carriers: Improving the Efficacy of Existing Cell Therapies
Although stem-cell therapies (SCTs) hold significant promise for treating chronic disease, a major difficulty in their clinical use comes with the high levels of cell death and low levels of cell retention in target locations after transplantation. Intravenous (IV) injection is a common and minimally invasive method of delivery for cell transplants (1). It may seem to be an ideal choice to provide large doses of cells, but IV transplantation is limited by insufficient homing, which leads to transplanted cells either dying without leaving circulation or becoming trapped in undesired organs (2, 3).
One way to address problems with viability and retention is to use supratherapeutic doses or repeated injections to ensure that sufficient cell numbers survive and reach their target region (4–6). However, high doses and serial injections not only exacerbate the issues of cost and scalability, but also introduce significant safety concerns (7).
Several biomolecular approaches to addressing transplanted cell retention and viability are under investigation (8–10). A number of biomaterials have been evaluated for their potential to aid in cell delivery (11). For example, Lin et al. reported enhanced efficacy of bone-marrow–derived (BMD) cell therapy in a postinfarction pig model when cells were encapsulated alongside peptide nanofibers (12). Another biomaterial-based strategy, reported by Kai et al., uses an electrospun poly(ε-caprolactone) and gelatin nanofibrous patch to assist in the delivery of mesenchymal stem cells (MSCs) into a myocardial infarction rodent model (13). Other approaches use stem cells themselves to engineer a biomaterial. Demonstrating the potential of cell-sheet technology, Ito et al. used BMD-MSCs to create a sheet composed of the cells with their extracellular matrix (ECM) proteins (14). However, hydrogel-based approaches represent the overwhelming majority of biomaterials used for cell delivery (15).
Hydrogels are crosslinked networks of hydrophilic polymer chains that are both insoluble in water and capable of absorbing large volumes of it (16). Their extensive use in the pharmaceutical and biomedical fields is due to their biocompatibility, biodegradability, tunability, and capability to mimic the ECM (17). One way to classify hydrogels is by the source of the polymer used to make them. Biopolymers can come from either synthetic or natural sources, and the material identity determines the properties of a resulting hydrogel. Both natural and synthetic polymers have advantages and disadvantages. With tunable and reproducible synthesis processes that provide for controlled physicochemical and mechanical properties, synthetic hydrogels are attractive for biomedical applications (18).
Natural polymers such as collagen, gelatin, and hyaluronic acid (HA) often are used to synthesize hydrogels for biomedical applications. The primary advantage of natural hydrogels is their biocompatibility. Common clinical uses include hydrogel-based wound dressings, cosmetic products, contact lenses, and drug delivery (18).
Our company focuses on hydrogels as cell encapsulants. Immunoprotection is one goal of such encapsulation that is especially of interest in treatment of type 1 diabetes. It is hypothesized that delivery of glucose-responsive, insulin-secreting cells inside hydrogels can bypass the need for frequent insulin injections (Figure 1, left panel). By sufficiently modifying a hydrogel network, encapsulated cells can release therapeutic compounds such as insulin while being protected from a host’s immune system.
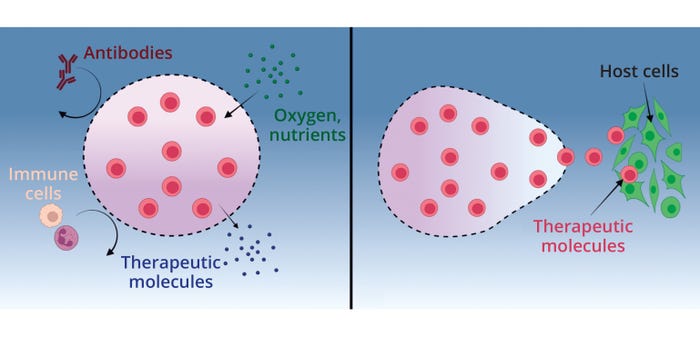
Figure 1: Hydrogels as cell encapsulants, (left) offering immunoprotection to encapsulated cells so that they can release therapeutic compounds from their network and (ri ght) facilitating release of encapsulated cells to interact with host tissues (19) (MADE WITH BIORENDER).
Cell encapsulation also has been used to facilitate local delivery and controlled release so that the transplanted cells can interact with host cells (Figure 1, right panel) (19). This particular use of hydrogels as delivery vehicles is beneficial for applications in which the therapeutic mechanism of transplanted cells is not limited to their secretions. In many situations, encapsulated cells must interact directly with surrounding tissues to have their intended effect. Cleverly designed hydrogels can provide local, sustained treatment for an array of disease states (19, 20).
Benefits of Hydrogels and Microspheres
Most current MSC-based therapies are delivered by syringe while suspended in saline or another appropriate medium (21). During administration, cells are exposed to high levels of shear stress that can disrupt their membranes and cause considerable cell death (22). In fact, some groups have reported that 3–40% of transplanted cells are killed during injection (23). As viscoelastic materials, hydrogels can absorb and dissipate applied forces. Encapsulation in a hydrogel before delivery can help mitigate cell death by reducing the shear forces during injection (22).
When stored in a low-viscosity solution such as a saline, adherent cell types can undergo a specialized form of apoptosis called anoikis (21, 22). It is caused by a loss of cell–ECM attachment regions and can be detrimental to the viability of a stem-cell transplant. Many cell types, including multipotent stem and stromal cells, are anchorage-dependent and require integrin interactions to survive. Cells communicate with their surroundings through focal adhesions, which are clusters of ECM-bound integrins that enable actin fibrils inside cells to attach (24). Failure to provide a substrate for attachment detrimentally impacts many cell-signaling pathways related to adhesion and apoptosis. For example, interaction of integrin-linked kinase with an ECM regulates adhesion-mediated cell survival (24, 25). When anchorage-dependent cells are released and transplanted as a suspension, they are deprived of matrix interactions — a situation that can cause anoikis. This apoptotic process can be prevented through the use of hydrogel delivery vehicles that provide encapsulated cells a physical substrate to which they can adhere (21).
Benefits of Hydrogel Microspheres: Microscopic hydrogels (microspheres) offer distinct advantages for cell encapsulation. Cell viability inside a hydrogel depends partly on the ability of nutrients and waste to diffuse in and out. Microspheres ideally facilitate that process by presenting a lower diffusion barrier than that of bulk hydrogels (26–28). Nutrients can enter cells in microspheres, and waste material can diffuse out with little diffusion barrier. Further, the implantation of microspheres is significantly less cumbersome and invasive than that of bulk hydrogels because an entire population of microspheres can be injected at once.
Characterization of Hydrogel Microspheres
Improving the efficacy of cell therapies through proper characterization of hydrogel carriers requires a thorough understanding and optimization of their properties. First, we need to examine and understand hydrogel properties, including the carriers’ physical, chemical, and mechanical attributes. We also evaluate the biocompatibility and biodegradability of hydrogels to ensure that they will be suitable for a given cell therapy.
A number of material properties affect a hydrogel microsphere’s ability to encapsulate cells successfully and deliver them in vivo. At Likarda, we use characterization data to customize hydrogel design — for example, by adjusting factors such as pore size, stiffness, diameter, and surface properties to enhance compatibility with specific cell types. The careful matching of a hydrogel to a planned therapeutic outcome can promote improved cell viability, proliferation, and functionality. In addition to altering the physical structure of a hydrogel, bioactive molecules or growth factors can be incorporated into the matrix to enhance therapeutic effects.
Physical characteristics to be measured include microsphere diameters and batch monodispersity. Chemical characteristics can include the polymer mass fraction; mechanical properties can be measured using rheology (26, 27). Biocompatibility and biodegradability are determined in vivo with rodent studies, as we have published previously (29).
Swelling Ratio: The swelling ratio (Q) is one of the most important characteristics of a hydrogel because it directly affects the material’s other properties. Calculated as the ratio of the swollen hydrated mass to that of the dry mass, Q is expressed either as the mass degree of swelling or the volume degree of swelling. This important value plays a direct role in the permeability of water and other compounds such as nutrients and waste, material degradability, and rheology — the importance of which is explained below.
Diameter: The diameter of a hydrogel microsphere has major implications for two factors that are important to the design of a successful delivery vehicle: First, the radius represents the distance that nutrients, waste, and other soluble factors have to travel to enter and exit the microsphere. Second, the diameter determines the size of needle or catheter that will be appropriate for in vivo injections, which has implications for clinical use.
Permeability: A cell-laden hydrogel’s permeability directly contributes to the therapeutic functionality of microspheres in multiple ways. The ability of nutrients and waste to permeate the material is essential to encapsulated-cell viability (30). Permeation through the microspheres of trophic and immunomodulatory secretions released from encapsulated cells is essential for indications in which efficacy relies on such secretions. And hydrogel degradation depends on the ability of degrading species to access molecular groups that are susceptible to their effects, often through diffusion. The degradation rate of a hydrogel determines the retention time of an encapsulated therapeutic at its target site.
Biocompatibility: Because failure to achieve biocompatibility can result in a failed cell therapy, this property of hydrogels is particularly important. Implantation of a foreign material into someone’s body induces protein adsorption on that material’s surface, prompting a survey by the person’s immune system that can lead to an inflammatory response (31, 32). If the materials are deemed to be hazardous, immune cells will attempt to degrade it by secreting reactive oxygen species and degrading enzymes. If such degradation fails, then a fibrous layer of tissue will be deposited around the material in an attempt to exclude it from interactions with the host (33). Having been “jailed” behind a wall of fibrous tissue, encapsulated cells no longer have access to nutrients and will die.
Biodegradability: The degradability of a hydrogel microsphere is important for fine-tuning encapsulated cell release. Degradation products must be minimally toxic, metabolizable into benign products, and/or excretable through renal filtration (34). If developers do not take care to engineer harmless biodegradation of a hydrogel microsphere, its degradation products can lead to death of transplanted cells and even their host.
Maximizing Therapeutic Potential
Based on the principles listed above, our company has established robust characterization protocols to ensure consistency and reproducibility of hydrogel properties across different batches. We implement rigorous quality control measures to maintain desired characteristics over time and use artificial intelligence (AI) to ensure that our cell-characterization assays are reliable and validated. Such measures include sterility testing and potency tests that depend on the cells being delivered. For example, if the payload were liver cells, then quality tests could include tests for secretion of albumin and cytochrome P450 (CYP) enzymatic activity.
To accelerate product approvals, we are navigating regulatory considerations by keeping abreast of requirements and standards for hydrogel-based cell therapies. Because guidance regarding inactive excipients for cell therapies is a completely new field, we collaborate with regulators to determine the needed data to support carrier safety. Characterization data must align with regulatory expectations.
Our goal is to facilitate the translation of laboratory findings to clinical applications —by conducting preclinical studies that validate the efficacy of hydrogel carriers in relevant disease models. We systematically characterize hydrogel materials for cell therapies, then leverage that knowledge to optimize their design, unlocking their full potential and ultimately contributing to advancements in the field of regenerative medicine. To customize hydrogel technology to each cell type, we evaluate how the carriers can maximize the therapeutic potential of specific cells, improving engraftment, tissue integration, and overall treatment outcomes.
References
1 Lapidot T, Dar A, Kollet O. How Do Stem Cells Find Their Way Home? Blood 106(6) 2005: 1901–1910; https://doi.org/10.1182/blood-2005-04-1417.
2 Li S, et al. When Stem Cells Meet COVID-19: Recent Advances, Challenges, and Future Perspectives. Stem Cell Res. Ther. 13(9) 2022; https://doi.org/10.1186/s13287-021-02683-1.
3 Ankrum J, Karp JM. Mesenchymal Stem Cell Therapy: Two Steps Forward, One Step Back. Trends Molec. Med. 16(5) 2010: 203–209; https://doi.org/10.1016/j.molmed.2010.02.005.
4 Lamo-Espinosa JM, et al. Intra-Articular Injection of Two Different Doses of Autologous Bone Marrow Mesenchymal Stem Cells Versus Hyaluronic Acid in the Treatment of Knee Osteoarthritis: Multicenter Randomized Controlled Clinical Trial (Phase I/II). J. Transl. Med. 16(213) 2018: 246; https://doi.org/10.1186/s12967-018-1591-7.
5 Matas J, et al. Umbilical Cord–Derived Mesenchymal Stromal Sells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 8(3) 2019: 215–224; https://doi.org/10.1002/sctm.18-0053.
6 Ozeki N, et al. Not Single but Periodic Injections of Synovial Mesenchymal Stem Cells Maintain Viable Cells in Knees and Inhibit Osteoarthritis Progression in Rats. Osteoarth. Cart. 24(6) 2016: 1061–1070; https://doi.org/10.1016/j.joca.2015.12.018.
7 Lukomska B, et al. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019: 9628536; https://doi.org/10.1155/2019/9628536.
8 Yin JQ, Zhu J, Ankrum JA. Manufacturing of Primed Mesenchymal Stromal Cells for Therapy. Nature Biomed. Eng. 3(2) 2019: 90–104; https://doi.org/10.1038/s41551-018-0325-8.
9 Wang H, et al. Genetically Engineered and Enucleated Human Mesenchymal Stromal Cells for the Targeted Delivery of Therapeutics to Diseased Tissue. Nature Biomed. Eng. 6(7) 2022: 882–897; https://doi.org/10.1038/s41551-021-00815-9.
10 Smith CL, et al. Pre-Exposure of Human Adipose Mesenchymal Stem Cells to Soluble Factors Enhances Their Homing to Brain Cancer. Stem Cells Transl. Med. 4(3) 2015: 239–251; https://doi.org/10.5966/sctm.2014-0149.
11 Wang X, et al. Advanced Functional Biomaterials for Stem Cell Delivery in Regenerative Engineering and Medicine. Adv. Funct. Mater. 29(23) 2019: 1809009; https://doi.org/10.1002/adfm.201809009.
12 Lin Y-D, et al. Intramyocardial Peptide Nanofiber Injection Improves Postinfarction Ventricular Remodeling and Efficacy of Bone Marrow Cell Therapy in Pigs. Circulation 122(11) 2010: S132–S141; https://doi.org/10.1161/circulationaha.110.939512.
13 Kai D, et al. Stem Cell–Loaded Nanofibrous Patch Promotes the Regeneration of Infarcted Myocardium with Functional Improvement in Rat Model. Acta Biomater. 10(6) 2014: 2727–2738; https://doi.org/10.1016/j.actbio.2014.02.030.
14 Ito M, et al. Application of Cell Sheet Technology to Bone Marrow Stromal Cell Transplantation for Rat Brain Infarct. J. Tiss. Eng. Regen. Med. 11(2) 2017: 375–381; https://doi.org/10.1002/term.1920.
15 Nadlacki B, Suuronen EJ. Biomaterial Strategies To Improve the Efficacy of Bone Marrow Cell Therapy for Myocardial Infarction. Expert Opin. Biol. Ther. 16(12) 2016: 1501–1516; https://doi.org/10.1080/14712598.2016.1235149.
16 Ahmed EM. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 6(2) 2015: 105–121; https://doi.org/10.1016/j.jare.2013.07.006.
17 Slaughter BV, et al. Hydrogels in Regenerative Medicine. Adv. Mater. 21(32–33) 2009: 3307–3329; https://doi.org/10.1002/adma.200802106.
18 Aswathy SH, Narendrakumar U, Manjubala I. Commercial Hydrogels for Biomedical Applications. Heliyon 6(4) 2020: e03719; https://doi.org/10.1016/j.heliyon.2020.e03719.
19 Gonzalez-Pujana A, et al. Cell Microencapsulation Technology: Current Vision of Its Therapeutic Potential Through the Administration Routes. J. Drug Deliv. Sci. Technol. 42, 2017: 49–62; https://doi.org/10.1016/J.JDDST.2017.03.028.
20 Vaithilingam V, Bal S, Tuch BE. Encapsulated Islet Transplantation: Where Do We Stand? Rev. Diabet. Stud. 14(1) 2017: 51–78; https://doi.org/10.1900/rds.2017.14.51.
21 Mooney DJ, Vandenburgh H. Cell Delivery Mechanisms for Tissue Repair. Cell Stem Cell 2(3) 2008: 205–213; https://doi.org/10.1016/j.stem.2008.02.005.
22 Baldari S, et al. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 18(10) 2017: 2087; https://doi.org/10.3390/ijms18102087.
23 Walker PA, et al. Effect of Needle Diameter and Flow Rate on Rat and Human Mesenchymal Stromal Cell Characterization and Viability. Tiss. Eng. Part C Meth. 16(5) 2010: 989–997; https://doi.org/10.1089/ten.tec.2009.0423.
24 Benoit DSW, et al. Integrin-Linked Kinase Production Prevents Anoikis in Human Mesenchymal Stem Cells. J. Biomed. Mater. Res. A 81a(2) 2007: 259–268; https://doi.org/10.1002/jbm.a.31292.
25 Ma Z, et al. Mechanotransduction and Anoikis: Death and the Homeless Cell. Cell Cycle 7(16) 2008: 2462–2465; https://doi.org/10.4161/cc.7.16.6463.
26 Hamilton M, et al. Hyaluronic Acid Hydrogel Microspheres for Slow Release Stem Cell Delivery. ACS Biomat. Sci. Eng. 7(8) 2021: 3754–3763; https://doi.org/10.1021/acsbiomaterials.1c00658.
27 Harrington S, et al. A Versatile Microencapsulation Platform for Hyaluronic Acid and Polyethylene Glycol. Tissue Eng. Part A 27(3–4) 2021: 153–164; https://doi.org/10.1089/ten.tea.2019.0286.
28 Sahu N, et al. Encapsulated Mesenchymal Stromal Cell Microbeads Promote Endogenous Regeneration of Osteoarthritic Cartilage Ex Vivo. Adv. Healthc. Mater. 10(8) 2021: e2002118; https://doi.org/10.1002/adhm.202002118.
29 Hamilton M, et al. Controlled-Release Hydrogel Microspheres To Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis. Bioengineering 10(11) 2023: 1315; https://doi.org/10.3390/bioengineering10111315.
30 Lee SH, et al. In Situ Crosslinkable Gelatin Hydrogels for Vasculogenic Induction and Delivery of Mesenchymal Stem Cells. Adv. Funct. Mater. 24(43) 2014: 6771–6781; https://doi.org/10.1002/adfm.201401110.
31 Anderson JM, Rodriguez A, Chang DT. Foreign Body Reaction to Biomaterials. Semin. Immunol. 20(2) 2008: 86–100; https://doi.org/10.1016%2Fj.smim.2007.11.004.
32 Swartzlander MD, et al. Linking the Foreign Body Response and Protein Adsorption to PEG-Based Hydrogels Using Proteomics. Biomaterials 41, 2015: 26–36; https://doi.org/10.1016/j.biomaterials.2014.11.026.
33 Carnicer-Lombarte A, et al. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 9, 2021: 622524; https://doi.org/10.3389/fbioe.2021.622524.
34 Li J, Mooney DJ. Designing Hydrogels for Controlled Drug Delivery. Nature Rev.Mater. 1(12) 2016: 16071; https://doi.org/10.1038/natrevmats.2016.71.
Dr. Stella Vnook is chief executive officer, Dr. Megan Hamilton was a senior scientist (now a technology analyst at Erise), and Dr. Lisa Stehno-Bittel is president and founder of Likarda, Inc., 10330 Hickman Mills Drive, Kansas City, MO 64137; https://likarda.com.
You May Also Like






