Comparability for Cell and Gene Therapy Products: A Challenge and an Opportunity
February 22, 2024
Cell and gene therapies (CGTs) are the fastest growing segment of biotechnology products, with
chimeric antigen receptor (CAR) T-cell products and gene therapies using adenoassociated virus (AAV) vectors dominating the landscape. To meet the increasing demands of these promising therapeutics, manufacturing changes often are implemented during clinical development as sponsors scale production up or out and optimize processes for productivity and performance. A well-designed comparability study is critical for demonstrating an absence of adverse effects on product quality, safety, and efficacy when a manufacturing change is made. The complexity of CGT product modalities and their current level of characterization pose challenges to the approaches typically used for assessing comparability, as guided by the principles described in ICH Q5E (1).
Comparability strategies that are tailored to each program and product type are key to successfully implementing manufacturing changes throughout product development and commercialization. Such adjustments can help streamline development, reduce cost of goods sold (CoGS), and ultimately increase access for a wider patient pool. Implementing changes to meet the demands of clinical supply and commercialization has presented distinct obstacles for CGT products. For example, transition from an adherent to a suspension-adapted cell culture process for manufacturing AAV vector-based products can increase output while increasing the level of process- and product-related impurities in final products.
For cell-based products, the inherent variability of cellular starting material introduces variability that can persist into a final product. That can make it difficult to distinguish the manufacturing process from the cellular starting material as the source of differences in final product quality. Many cell-based products are made to order for single patients (autologous products). In such cases, limited material availability for analytical evaluation creates constraints on comparability study designs. Similar issues must be addressed in planning comparability studies for viral-vector–based gene therapy products in development for rare diseases.
Overall, the complex manufacturing processes for CGT products combined with currently limited understanding of clinically relevant product quality attributes (PQAs) make it important to design a “fit for purpose” comparability approach. Developers can benefit from discussions at forums such as CASSS meetings for sharing of knowledge, novel technologies, experience, and case studies that can improve understanding of how guidance from regulatory agencies can be applied in managing manufacturing changes and expediting the delivery of life-saving products to patients. In its inaugural one-day Cell and Gene Therapy Products (CGTP)summit held 26 June 2023, CASSS brought together experts from industry, academia, and regulatory agencies to discuss challenges and opportunities in the design and implementation of comparability strategies for CGT products. This summit was held just before the US Food and Drug Administration’s (FDA’s) release of a draft comparability guidance in July 2023 (2).
The morning session of this summit was devoted to viral-vector–based gene therapies; the afternoon session was focused on cell-based gene therapies. Each talk in the morning session was followed by a panel discussion in which experts fielded questions from online and onsite audiences. The presentations and the panel discussions focused on comparability studies for AAV-based gene-therapy products and CAR T-cell therapies, with related statistical considerations for study design. Learnings from the first developed AAV-based and CAR T-cell products can be applied to other product types in the CGT family that will be advancing rapidly toward commercialization in the near future.
In an introduction to the summit, the chairperson noted that the overarching principles of comparability assessments described in the ICH Q5E guidance should be applied to CGT products using a risk-based approach. Notably, comparability does not necessarily mean that the quality attributes of prechange and postchange material will be identical, but rather that they are highly similar and that the existing knowledge is sufficiently predictive to ensure that any differences will have no adverse effects on safety or efficacy. When considering a manufacturing change, a product developer should consider its potential influence on other parts of the process and on product quality — and should assess the potential risk of adversely affecting product safety or efficacy.
The following points also were highlighted: Determination of comparability can be based on a combination of analytical testing, biological assays, and in some cases, nonclinical and clinical data. Comparability assessments rely on a body of knowledge, and product and process understanding are important. CGT comparability studies present some difficulties given the current level of maturity of the field, and some of the flexibility needed for such products is beyond what is currently addressed in ICH Q5E. And finally, discussions are needed among developers and regulators to overcome the current limitations for each CGT product modality.
Morning Session
Comparability Considerations for Viral-Vector–Based Gene Therapies: Demonstrating comparability for viral-vector–based gene therapies has become more straightforward than that for cell-based products, in part because standard manufacturing technologies (e.g., chromatography and filtration) are used and improved analytical testing methodologies have been developed. Advancements in the elucidation of structure–function relationships for AAV-based gene therapies have been critical in enabling additional characterization studies to support manufacturing changes.
Presented case studies made it clear that effectively demonstrating comparability relies on an in-depth understanding of a given product, on well-designed structure–function studies, and on reliable approaches to measure product quality and potency. One case study described substantial manufacturing changes made early in development to increase yield and scalability. Those changes included switching from a human-based adherent-cell culture system to an insect-cell–based suspension-cell culture system for vector manufacturing and certain downstream process changes for product purification. They were all major changes, which when implemented at the same time would make demonstration of comparability difficult. To implement those major changes successfully during early clinical development — and to mitigate the related risks to quality attributes — a comparability strategy was planned and executed. It focused on a matrixed approach that included the following elements:
• analytical comparisons that included release, characterization, and stability testing
• animal studies including nonhuman primate (NHP) toxicology studies to address potential impact to product safety and a head-to-head mouse study to address product efficacy/dose response before and after changes were made.
Limited analytical method-development concerns were addressed in that case, with side-by-side testing of pre- and postchange product and orthogonal testing for confirmation. Additional problems included limited availability of test materials, which was addressed by supplementing with data from process development (PD) lots generated under conditions that were not compliant with good manufacturing practices (GMPs). Such an approach aligns with recommendations in the draft comparability guidance issued by the FDA in summer 2023 (2).
A second case study reported on changing from a serum-dependent adherent cell culture to a suspension-adapted, serum-independent cell culture system for vector manufacturing in support of late-phase development and future commercial needs. That required rederivation of cell banks and simultaneous changes to downstream process steps, manufacturing scale, and manufacturing site. Such major manufacturing changes made during late stages of clinical development were derisked through an analytical comparability strategy that was grounded by historical process and product knowledge, with a testing plan that applied an advanced analytical toolbox. This case study also included strategies to adopt analytical release method changes accompanying the planned manufacturing changes. The presenter showed that adequate analytical method bridging studies can support the introduction of new assays late in product development for future quality control (QC) testing of late-stage and commercial lots.
Note that health authorities encourage developers to use precise, accurate, and sensitive assays that leverage current technological advances — e.g., moving from quantitative polymerase chain reaction (qPCR) to Bio-Rad’s droplet-digital PCR (ddPCR) technology for product testing and demonstration of comparability. Applied assays should be scientifically sound and appropriate both for their intended purpose and the stage of clinical development. For late-phase studies, a qualified/validated activity-based potency assay should be in place for a comparability study.
Central to the demonstration of comparability is defining a meaningful difference between data from pre- and postchange products. Selecting appropriate statistical approaches to demonstrate a meaningful difference is often a vexing issue among developers of CGT products. In one presentation, that topic was approached holistically by highlighting that the assessment of comparability is more than “just numbers.” The choice of statistical approach depends on the question that is asked. In practice, that would mean knowing what will be compared and how potential changes to product attributes will be measured. Whether to use a robust statistical methodology or descriptive summary statistics (describing sample size, mean/median, data spread/distribution, comparisons of graphed results, and so on) should depend in part on the size of data sets available. Generated manufacturing experience, which generally depends on the phase of clinical development, is likely to affect the choice of statistical approach for analyzing comparability data. The presenter emphasized the importance of considering all available data (including PD data) in the comparability analysis and health authorities with access to such data.
Together, the case studies and statistical considerations presented in the morning session underscored the value of comprehensive, multifaceted comparability studies for viral-vector–based gene-therapy products. The extent of a study and its design are influenced by
• the type of (major or minor) manufacturing changes planned
• associated risk assessments
• the (early or late) phase of development
• product and process understanding
• available analytical tools (and their suitability for detecting potential changes in product attributes)
• the appropriate statistical approach
• the extent of data available to demonstrate comparability (when studies will be conducted).
A stepwise approach should be adopted. One of the first steps in assessing the potential effects of a manufacturing process change is identification of product attributes that are likely to be affected by the planned manufacturing change(s). The resulting list of attributes then should guide design of a comparability study. For CGT products, a robust potency assay that demonstrates the mechanism of action (MoA) could be the most powerful tool to establish a correlation between patient outcomes (safety and efficacy) and PQAs. However, even with a reliable potency assay in place, demonstrating analytical comparability alone may be inadequate to ensure that pre- and postchange products will show similar safety and efficacy profiles in vivo. In some cases, regulators might request that additional in vivo studies be conducted — for example, if there is a reliable animal model to assess dose-response curves with pre- and postchange products. The discussion panel noted that nonclinical studies generally are less precise than analytical methods and in some cases actually could provide less valuable information than an in vitro assay would.
Afternoon Session
Comparability Considerations for Cell-Based Therapies: When it comes to applying the principles of comparability assessments as described in ICH Q5E, cell-based therapies present unique challenges for several reasons, including multifaceted manufacturing processes and the complexity of cells and the MoAs of cell-based products. Current understanding of the PQAs for cell-based therapies is limited, so it is difficult to identify which attributes would be relevant to product safety and efficacy. From a regulatory perspective, many cell-based therapies are highly variable by nature and thus can pose challenges for assessing manufacturing consistency and evaluating comparability to ensure that every patient receives a product of sufficient quality. Regulators recommend that product and process characterization and assay development begin early in a program and continue throughout a product’s life cycle. Comparability study design depends on the risk posed by a given change, the stage of development at which it is made, and the potential effects on PQAs and interpretation of clinical study data.
Patient-derived starting materials are particularly heterogeneous for autologous cell-based therapies. Variability in cellular starting material affects both process performance and lot-to-lot product quality, so comparability studies need to account for that. For example, a known concern with autologous, lentiviral vector (LVV)–transduced CAR T-cell products is variability in their expression of CAR protein — not necessarily because of differences in the LVV, but rather in the incoming T cells themselves. Differences in the quality of starting material from patients are the primary reason why the length of a commercial CAR T-cell manufacturing process can be highly variable among patients (taking from one to three weeks). Such high variability in process duration affects CoGS, forecasts per run, and availability of manufacturing suites — and it ultimately can delay the availability of drug products for patients in advanced stages of disease.
A well-designed comparability package should use correlative analyses to parse out the contribution of starting-material variability from minor differences in product quality. In addition to building product understanding, such exercises in correlative analysis prepare a sponsor to present regulators with a data-driven case to address minor changes in product quality, which is not unusual with autologous cell-based therapies. Similar challenges are observed for allogeneic, donor-derived cell-based therapies, with which variability among donors (or even between donations from a given donor) affects the profile of cellular starting material and thus final product outcomes.
An afternoon case study provided a solution to account for patient/donor starting material variability. “Blocked” or paired-run studies (also known as split manufacturing runs) divide a manufacturing process at the cellular starting material (or further downstream in the process, depending on the nature of a change). Such studies using the same cellular starting material can help a sponsor establish comparability by removing patient/donor variability from the assessment (Figure 1). For autologous cell-based therapies, it might be necessary to use healthy donor cells as a surrogate starting material when patient-derived material is not available for such studies. This case study also emphasized the need for early planning of comparability studies and minimizing the number of studies necessary by addressing more than one process change at a time. When manufacturing changes are implemented during early clinical study phases, comparability studies can focus on demonstrating no adverse effect on safety.
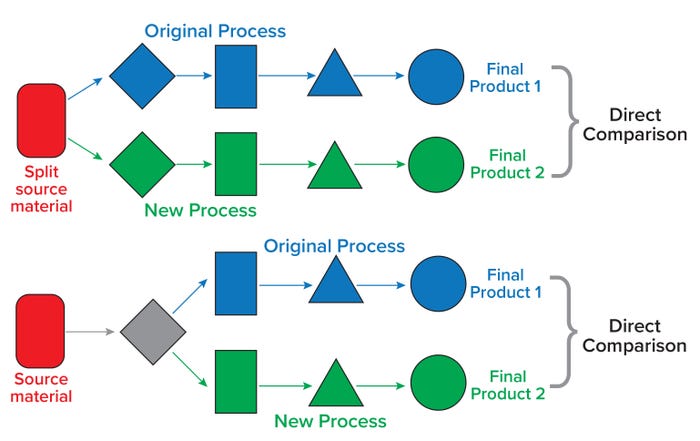
Figure 1: Paired study runs from the same donor material can reduce the complexity in designing comparability studies. (top) A typical paired study requires split postsource material because the intended change is introduced very early in the manufacturing process. (bottom) In another paired study design, a paired split is introduced to streamline comparability after an intermediate step(s) (gray diamond). Keeping the donor constant in either case removes patient/donor variability from a comparability perspective.
In some cases, when significant changes are introduced after initiation of pivotal clinical studies, sponsors might have to expand the clinical trials to gather enough data from patients who received postchange products. That might be necessary for a true assessment of the impact on both the safety and efficacy of those products. Such expansions can increase costs, delay timelines, and make commercializing cell-based therapies fiscally unsustainable.
Learnings from the development of autologous cell-based therapies can be leveraged in development of allogeneic therapies, particularly regarding what constitutes a “better” starting material for manufacturing multiple batches from a small pool of donors. In manufacturing of T-cell products, early memory phenotypes, less differentiation, and overall cell health are generally considered to be critical for success. For ensuring consistent and successful manufacturing, such learnings also can be leveraged in selecting donors for allogeneic therapies.
At the summit, robust debate ensued about the proper use of statistical modeling in comparability assessments for cell-based therapy products. During the panel discussion, some limitations were pointed out about implementing statistical models for comparability assessments of cell-based products. In particular, their characterization is still evolving, so justification is currently inadequate for including potential critical quality attributes (CQAs) in statistical modeling. In addition, data sets might be too limited to be statistically meaningful.
Because CGTs are being developed for a global market, alignment is needed across regulatory agencies with regard to proper use of statistics and statistical modeling for demonstrating comparability of CGT products when manufacturing changes are made. Successful implementation of an analytical comparability study requires comprehensive understanding of quality attributes and a product’s MoA. It is important to recognize the CGT industry’s current level of maturity when discussing a “comprehensive” understanding of a product. The relatively limited understanding of CGTs compared with traditional recombinant-protein products is the primary barrier to developing an analytical approach for assessing comparability. Meaningful measures of potency in analytical comparability studies are crucial to verifying that in vivo activity is not compromised.
For now, a “fit for purpose” approach is needed for CGTs, given the limited product and process understanding combined with the necessity of manufacturing process changes. Building up comprehensive product understanding for cell-based therapies will require dedicated organizational efforts to integrate process and product knowledge. Many companies have a dedicated function to consolidate data from QC and characterization methods together with research and nonclinical studies as well as clinical samples to elucidate functional effects based on correlational analysis. Building a deep understanding of advanced therapies is likely to involve an extended learning process.
Leveraging deep CGT product understanding will be key to the future of comparability strategies for
• assessing the true impact on product quality before and after manufacturing changes
• defining the needed analytics to capture accurately the effects of proposed manufacturing changes
• ascertaining and ensuring that product coming from a new manufacturing process is highly similar to what has been evaluated in patients already — and that the existing knowledge is sufficiently predictive to ensure that any differences in PQAs have no adverse effect on product safety or efficacy.
CGTs are some of the most complex drugs developed and manufactured to date. As discussed, complexities in the MoA and manufacturing of these products makes the impact of process changes on safety and efficacy difficult to quantify precisely. However, manufacturing changes must be made during development. Thus, the commercial success of CGTs will rely heavily on well-designed comparability exercises that can address the effects of manufacturing changes adequately using information available now. Advanced-therapy (CGT) sponsors should continue to build the analytical tools necessary to gain a deeper understanding of these products to enable future development.
References
1 ICH Q5E. Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process. US Fed. Reg. 70(125) 2005: 37861–37862; https://database.ich.org/sites/default/files/Q5E%20Guideline.pdf.
2 CBER. Manufacturing Changes and Comparability for Human Cellular and Gene Therapy Products. US Food and Drug Administration: Rockville, MD, July 2023; https://www.fda.gov/media/170198/download.
K.R. Poudel is director and head of product development at Tune Therapeutics. Zenobia Taraporewala is a senior advisor in regulatory CMC at BioMarin Pharmaceuticals. Diane Blumenthal is principal consultant at Dianthus Biopharma Consulting, LLC. Deep Shah is technical regulatory program director for cellular therapies, and corresponding author Kathy Francissen is global head of regulatory for cell and gene therapies at Genentech (a Member of the Roche Group) in South San Francisco, CA; francissen.kathy@gene.com.
You May Also Like






