Designing the Optimal Manufacturing Strategy for an Adherent Allogeneic Cell TherapyDesigning the Optimal Manufacturing Strategy for an Adherent Allogeneic Cell Therapy
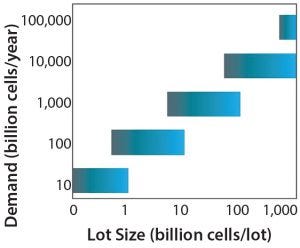
Figure 1: Recommended range of manufacturing lot sizes across different demands. Vertical axis shows examples of different demands. For each level of demand, the horizontal bars show the range of recommended lot sizes. This figure was generated under the assumption that the minimum and maximum numbers of lots that a given facility could produce in a year are 10 and 200, respectively (5).
Cell therapies (CTs) offer potential treatments for a wide range of medical conditions (1–6) by replacing cells, repairing tissues affected by either disease or damage (7), or delivering genetic or molecular agents that promote self-healing (8). CT research and development is continuously growing (9), with increasing numbers of CT candidates reaching phase 3 clinical trials (9–11). Developers aim to make products that can survive in a competitive landscape while complying with stringent regulatory requirements to control the quality and safety of their therapies (12). Successful commercialization requires careful alignment of a company’s business model with its manufacturing strategy (13).
An allogeneic cell therapy (allo-CT) uses cells from a universal donor to treat multiple patients. This makes manufacture amenable to scale-up, which confers advantages such as economies of scale and spreading the cost of goods (CoG) over several doses (14, 15, 5). Allo-CT developers are facing increased pressures for cost-effective manufacturing, especially for high-dose indications with large market potential (5). But because cell therapies are different from traditional biopharmaceuticals and small molecules (5, 9), several factors can affect the development of robust and cost-effective manufacturing processes for allo-CTs.
Here we outline key business considerations when planning the commercialization of a novel allo-CT product and the importance of taking product characteristics, manufacturing process parameters, and business risks into account.
Designing the Optimal Manufacturing Strategy
A manufacturing strategy must be developed and incorporated into a company’s business plan. It will determine the resources required for process development and manufacture. Those factors must be tensioned against a specified budget and available manufacturing capacity as well as a company’s attitude to risk.
Early Planning for Commercial Success: Designing a scalable manufacturing strategy as early as possible is paramount to a successful commercialization. Doing so helps allo-CT manufacturers cost-effectively produce sufficient quantities to meet demands at each stage of a clinical program and for commercialization.
Product bioequivalence must be established and demonstrated upon scale-up or technology transfer and with each process modification. That is particularly challenging for cell therapies because proving bioequivalence of critical quality attributes can be difficult with existing characterization assays (16). Making process changes also carries the risk of having to repeat clinical trials, adding time, risks, and costs to an already expensive, complex, and lengthy development process. Hence, early planning of a manufacturing strategy that is amenable to scale-up is key to meeting clinical and commercial demands on time, keeping manufacturing off the critical path, and achieving cost control.
Short-Term Goals and Long-Term Strategy: Historically during process development, the primary goal has been to progress a therapy into clinical trials. This may impair the commercialization of allo-CT products because processes with limited scalability (e.g., multilayer stacks) may lack the capacity (17, 18) and/or adequate automation to deliver large-scale commercial processes that are compliant with current good manufacturing practices (CGMP).
Some CT companies assume that future funding by an established sponsor — or an outright purchase of their product — will solve their manufacturing challenges before large-scale production is required. In some cases, they are not only unprepared to provide a CGMP-type validation package, but they have not seriously explored an appropriate large-scale manufacturing solution. Such a business strategy may no longer be feasible because larger, well-established sponsors are looking increasingly to smaller organizations for detailed plans about their commercialization strategies before investing.
Keeping Costs in Mind: In addition to meeting requirements for reproducibility, safety, and efficacy, a therapy must be cost effective (19). Offering a therapy at an affordable price increases its acceptance by both public and private healthcare providers. Price determinants for therapies can include patentability, reimbursement, lifecycle issues, demand elasticity, market competition, and manufacturing CoG.
The bioindustry is facing pressures to lower CT manufacturing costs to enable feasible business models and widen product accessibility. Prioritizing cost-reduction efforts requires a holistic evaluation of process economics to determine key cost drivers and bottlenecks. Factors such as capital investment, operating expenses (e.g., materials, labor, quality control), overhead costs, and market dynamics (market demand, market fluctuations, competition, and dosage ranges) need to be analyzed. Additional elements to consider include pricing constraints (which may vary because different countries will have distinct reimbursement policies) and gross profit margin targets. Those last two factors ultimately will set a target for manufacturing CoG/dose that allows for a gross margin large enough to recover R&D costs while still allowing sufficient profit. When evaluating various manufacturing options and choosing the ultimate manufacturing platform, it is important to consider manufacturing scale, process schedules, logistical aspects (e.g., shipping and handling), and regulatory implications. For example, manual processes are more prone to batch-to-batch variation, and regulatory approval of such processes at commercial scale may be more challenging then for automated processes.
Keeping CoG as low as possible facilitates delivery of a therapy competitively once commercialization is reached. Moreover, doing so will provide the flexibility to adjust a selling price as competitors enter the market.
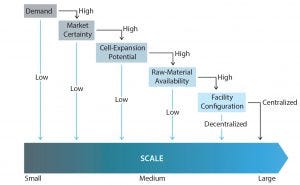
Figure 2: Decision tree shows the decision-making path for an optimal lot size
Determining the Ideal Lot Size
Process scale (and hence lot size) is central to a manufacturing strategy because it significantly affects the CoG. Lot size is typically measured in terms of doses or cells per lot. Additional factors affecting manufacturing CoG include product characteristics and business aspects, which must be considered when selecting the lot size and manufacturing platform.
Business Strategy Considerations: Two critical factors to consider when developing an allo-CT manufacturing strategy are the expected demand for a product once it is commercialized and market uncertainties. Doing so will allow manufacturers to design a process of adequate capacity and (at the same time) predict consequences of possible market deviations.
Market Potential: Characteristics of a target indication (e.g., cells per dose, administration schedule, administration term, and number of patients) will help a company predict overall market demand for a given allo-CT product. Other market forces include competing treatments and general acceptance of this novel class of therapies.
Published cost analyses have defined ideal lot sizes according to market demand (5, 20). Figure 1 highlights typical lot-size ranges for different demands. For allo-CTs, demands in the range of 10–100,000 billion cells per year can be envisaged based on doses reported and potential market sizes for indications ranging from age-related macular degeneration to liver disease. Such demand translates into lot sizes of 0.1–1,000 billion cells per lot. Lot size also will be influenced by the choice of whether to adopt a scale-out or scale-up strategy.
Market Certainty: If market certainty is low (perhaps because the market is new or highly competitive), using a scale-out model with a smaller-scale platform may be a better strategy. Such an approach would require lower upfront costs, which can be significant particularly for small companies. A scale-out model also provides some risk mitigation in the event that market capture does not meet expectations.
If demand is much larger, a scale-up strategy may seem more appropriate because CoG decreases with increasing scale (5, 20). For this case, however, several other factors need to be examined to determine the optimal lot size. If demand is high but certainty is low, adopting a medium-scale production platform between 1 billion and 50 billion cells/lot would help mitigate risks if actual demand is lower than predicted. But if both demand and certainty are high, then a manufacturer can choose the most cost-effective option: a large-scale production platform (e.g., 100 billion cells/lot).
Time required for sales to ramp up and reach maximum expected market penetration is another important aspect. If a production scale is designed to meet demand at peak sales, then a facility can be idle for extended periods during the first several years postlaunch, leading to high overhead costs. A possible solution to that predicament is to design a smaller scale process with enough flexibility to be easily expanded to accommodate higher demands as they arise.
Manufacturing Process Considerations: Process Robustness: One ramification of poor process robustness is lot failure. The consequences of lot failure should be investigated as part of deciding on an optimal lot size. If the process is well optimized and understood, the lot failure rate reduces to a minimum. However, it is still important estimate the “value” of a given lot by considering factors such as raw material availability, time constraints, and costs of media and other process components. Such an assessment is particularly critical when companies use organs with limited availability as the starting material because they are difficult to attain.
If large lot sizes (enough to cover six months’ worth of projected demand) are manufactured, then one failed lot could lead to a major financial loss, including sunken costs and costs of rerunning the lot. If time constraints or raw-material availability cause delays in meeting demand or inability to repeat the lot, then loss of revenues and potential damage to the brand are additional risks.
A small lot size (e.g., enough for one month’s demand) entails considerably smaller risks of failing to meet market demand. But this strategy will incur a higher CoG. One major contributor toward that increase is the cost of quality control (QC) because QC tests must be carried out on each lot.
Process robustness can be a driver for CT developers to engage contract manufacturing organizations (CMOs) for translating their benchtop processes into robust and cost-effective commercial-scale processes. The decision between in-house and CMO manufacture requires consideration of several factors:
Infrastructure of existing facilities and capabilities
Current in-house facilities
CMO expertise, scale of production, and availability
Laboratory to CMO technology transfer
Confidentiality implications
Loss of control over a manufacturing process
Time and costs of building an in-house facility compared with CMO costs.
Facility Configuration — Centralized or Decentralized?: Final lot sizes also will be determined by whether a centralized or decentralized facility configuration is used. A single manufacturing facility is nearly always the lowest-cost option (21); however, this approach increases the risk of a disruption in supply in the event of a production failure or unplanned shutdown. Risks associated with centralized production are magnified for products that must be shipped fresh. In such scenarios, lessons can be learned from the blood industry, in which fresh blood is transported around the world from centralized facilities with little supply disruption.
Using a centralized facility might present regulatory challenges because regulations differ among countries. A decentralized approach, however, offers a better option for risk mitigation in terms of both manufacturing failure and regulatory constraints. If a problem occurs at one facility, then market demand can still be met by increasing production in the remaining facilities. Moreover, a decentralized approach helps to minimize logistical issues associated with shipping cells globally and on time, and it reduces the costs of doing so. Potential disadvantages to this model include a requirement for greater oversight of process consistency and training across multiple sites (21). Those issues can be addressed by using fully automated platforms requiring very little training, thus reducing operator-related variability.
Product Considerations: Biological Constraints and Considerations: Individual characteristics of a selected cell type should be carefully considered when developing a manufacturing strategy. The cells’ proliferation rate and availability (starting material) play very important roles in selecting a manufacturing scale and vary according to the cell source (22).
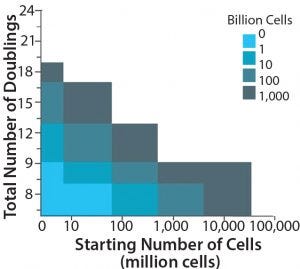
Figure 3: Maximum achievable lot size in cells per lot for each number of cells inoculated– total number of doublings combination; the starting number of cells is the initial number of cells retrieved from a donor. The white region indicates maximum achieved lot sizes >1012 cells/lot.
For example, mesenchymal stem cells (MSCs) have limited expansion potential. Therefore multiple cell banks may be required for large-scale cell culture (e.g., at the 1,000-L scale). Those can have cost, risk, added process variability, and regulatory implications. To generate master and working cell banks, cells must undergo cell-culture stages. Those stages will affect cell proliferation capacity because MSCs can undergo only a limited number of expansion stages without compromising the quality of a final product. Figure 3 shows how many cells can be produced, depending on initial number of cells retrieved from a donor source and maximum number of doublings that cells can undergo.
Product Final Form: A final product can be shipped frozen and reconstituted at the point of care or shipped fresh and ready to use from a manufacturing facility (21). If the latter, then the shelf life of the product is short, which restricts batch size to the number of cells required to treat a number of patients within a short timeframe. If a product is to be shipped frozen, then much larger lot sizes can be produced because the shelf life is now extended up to several years. Although frozen products allow for easier planning and more flexibility in the choice of manufacturing scale, there is a trade-off between increased supply-chain flexibility and potentially lower process yields.
Ideal Manufacturing Platform: Optimal CoG
Many CT products are developed with technologies that are not scalable. Over the years, a number of different technologies for expanding CTs have become available, each with different capacities, advantages, and disadvantages (5, 17, 20, 23, 24). Manufacturers must select the appropriate technology for their processes.
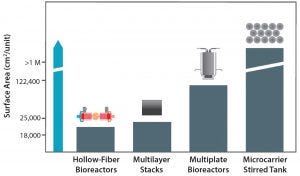
Figure 4: Available upstream technologies for adherent cell culture (25)
Upstream Manufacturing Technology Selection: Figure 4 shows available technologies for expansion of adherent cells in order of increasing surface area per cell culture vessel. CT research and development traditionally begins using open, two-dimensional (2D) multilayer stacks. However, that method is not practical for late clinical or commercial production because the number of culture flasks required for a single lot rapidly becomes unmanageable (24). Thus, multiple manual aseptic operations are required that would mandate very large manufacturing facilities and large numbers of operators (21, 25), hence increasing CoG. And the multitude of manual interventions required using this type of technology increases the risk of operator-related errors (26), presenting operational, quality assurance, and quality control problems.
With newer regulatory-compliant systems now available, some challenges associated with producing increasingly larger lots of cells are being overcome. One such system is the Xpansion multiplate bioreactor from Pall Life Sciences (18, 24). The Xpansion system is a compact, closed, single-use bioreactor developed for efficient, commercial-scale production of traditional 2D shear-sensitive cell cultures. This bioreactor provides the same microenvironment for cell growth as multilayer stacks, allowing easy transition from traditional open models to closed and controlled systems with minimal adaptation required. Thus, it is a viable larger-scale alternative to multilayer vessels. It is the only planar bioreactor with up to 122,000-cm² growth surface per single unit (24, 25), so it requires fewer manual operations than for cell factories.
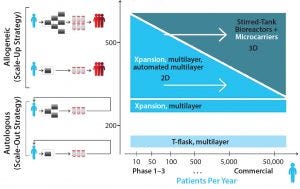
Figure 5: The relationship between increasing demand and technology availability, taking into consideration the number of patients to be treated each year (horizontal axis) and the number of cells/patient or dose size (vertical axis) (25)
The optimal technology selection should be carried out with the target yearly demand in mind, taking into account cell losses throughout the process. Figure 5 shows different technology options for adherent cell culture, according to the number of patients to be treated each year and dose size.
Open multilayer stacks typically are used for manufacturing low-dose products. Increasing production capacity involves scaling out rather than scaling up. Using bioreactors and/or automation will contribute to reducing CoG. Microcarriers in stirred-tank bioreactors (far right of Figure 4) are rapidly emerging as the premier platform for large-scale expansion of adherent cells. They are being used by many advanced CT companies in various stages of allo-CT development. Microcarriers are small-sized spheres — normally 90–300 μm in diameter (24) — that enable anchorage–dependent cell types to be grown in a 3D bioreactor system. Several processes use surface chemistry or coatings that promote attachment and growth of anchorage-dependent cells in cell culture procedures (24). A primary benefit is that they provide large rations of surface area to volume (4), allowing large-scale, cell-dense systems to fit within a relatively small footprint. This technology is scalable to very high volumes, enabling production of adherent cells into the millions of cells per milliliter and reaching scales of up to 1013 cells/lot (5, 23).
Pall SoloHill microcarriers were designed for large-scale expansion of adherent cells. These microcarriers require minimal preparation and offer consistency in performance, high recoveries, a range of surfaces with novel attachment/detachment properties, and animal-protein–free options for use in CT applications (24).
Transitioning from a static 2D to a 3D system and scaling it up requires time and expertise (25). These considerations should be factored into a company’s commercialization plans and timelines. Regulators will require assurance that products remain comparable in both production systems. Hence, adequate quality control procedures (including appropriate assays) must be developed and characterized before transition from 2D to 3D manufacturing.
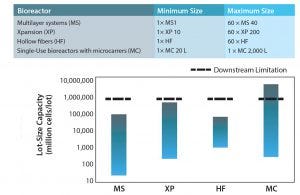
Figure 6: Capacity interval for different expansion technologies and current downstream (DSP) limitations in CT bioprocessing using a single kSep6000s unit under 4 hours. The solid bars represent the expansion potential of each technology, and the dashed lines represent the DSP limitations in each scenario. Assumed harvest density in all scenarios was 45,000 cells/cm2. A surface area per liter of 11,080 cm2/L was assumed in microcarried-based scenarios. MS = multilayer vessels, XP = multiplate bioreactor (Xpansion), HF = hollow-fiber bioreactor, MC = microcarrier-based bioreactor.
Overcoming Downstream Technology Gaps: Although more scalable technologies for expanding adherent cells are becoming available, significant gaps remain in harvesting, isolating, concentrating, washing, formulating, and cryopreserving when large cell culture volumes are processed (16). Hence downstream processing plays an important role in determining the production scale and thus should be carefully aligned with the upstream manufacturing scale.
Harvesting cells grown in adherent conditions from reactors and microcarrier systems typically requires enzymatic digestion of their adhesion proteins (17, 21, 23, 27), often through the use of physical force. The challenge in adopting existing downstream processing technologies for CTs is that the cells are living organisms and therefore highly sensitive to downstream process conditions and time (downstream processing windows are usually 1–4 hours) (16, 17, 21).
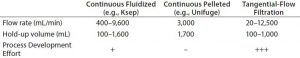
Table 1: Impact of column diameter on column weight, bed volume, and slurry tank size
Table 1 lists some common technologies used to wash and concentrate cells and their respective flow rates and throughputs. Continuous centrifuge systems are the closest to classical batch centrifuges, requiring the least amount of process development effort. Such systems, however, do not have enough capacity to handle a commercial-scale process (Table 1) (28). Alternatively, low continuous fluidized centrifuges and tangential-flow filtration (TFF) systems feature higher flow rates and low hold-up volumes. But because they are relatively new, such systems require a greater development effort to be used robustly in CT applications. Automated fluidized-bed centrifugation systems can concentrate, separate, and wash lot sizes as large as 100 billion cells (16, 28). Formulation additives generally are used before product formulation and filling.
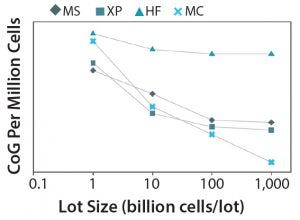
Figure 7: CoG per million cells with increasing annual throughput across multiple technologies; MS = multilayer stacks, XP = multiplate bioreactor (Xpansion), HF = hollow fiber bioreactor, MC = microcarrier-based bioreactor
Impact of Manufacturing Scale on Cost-Effectiveness of Upstream Technologies: A study from University College London’s Department of Biochemical Engineering provides several insights derived from an advanced bioprocess economics tool designed by its decisional tools team (20). Results suggest that at smaller scales of 1 billion cells/lot, multilayer vessels are the optimal technology to be used. At larger scales (1,000 billion cells/lot), microcarriers have presented significant cost savings with respect to remaining technologies (Figure 7).
Suitability of different technologies will depend on the production scale and experimental protocol used. The discussion below provides an insight into the distribution of current technologies across the different process scales.
Small Scale (1–10 billion cells/lot): Multilayer stacks offer a straightforward and easy solution for small-lot production. Ideal for R&D and pilot phases, these open systems do not provide full monitoring and regulation of culture conditions, and they require multiple manual operations or extensive automation. So multilayer stacks are less reliable for consistent, reproducible CGMP manufacturing. Hollow-fiber bioreactors represent a good alternative to minimize operation workload, but they can be cost-prohibitive for larger-scale applications.
Medium to Large Scale (10–100 billion cells/lot): Single-use bioreactors substantially reduce overall costs and simplify quality assurance (QA) and QC functions because they decrease the number of operations needed and allow for larger lot sizes to be achieved. However, most existing disposable bioreactors are designed for viral or protein production. Hence they are not suitable for expanding fragile adherent cells. New bioreactors, (e.g., the Xpansion multiplate bioreactor) allow for a fully closed process, guaranteeing safety and sterility during cell culture processing. Switching from multilayer stacks to the new type of single-use bioreactors does not affect the quality or nature of cells and reduces the complexity and risks of the scale-up process.
Implementing microcarriers allows users to take their bioproduction processes to much larger scales. Microcarriers support dense cultures at larger scales within a relatively small manufacturing footprint, and their use can lower cell culture production costs compared with using traditional cell culture methods. That cost advantage increases as processes are scaled up to larger production volumes. However, we reiterate that developing a microcarrier process can require a significantly higher level of development time and expertise (25).
Process Development Resources and Time: Often overlooked is identification of available resources for and risks of bioprocess development and manufacturing during early stages of process development. Accurate estimation of the resources required throughout a whole business lifecycle companies must consider
Process development costs and associated timelines
Anticipated cash flow
Immediately available assets
Facility investment and operating costs.
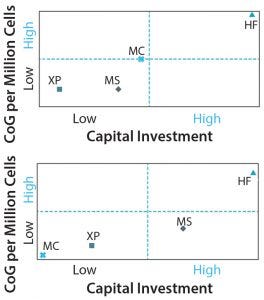
Figure 8: CoG per million cells versus fixed capital investment with increasing manufacturing scale for (top) a lot size of 10B cells and a demand of 1T cells per year and (bottom) a lot size of 100Bn cells and demand of 10T cells per year. MS = Multilayer stacks, XP =multiplate bioreactor (Xpansion), HF = hollow fibre bioreactor, MC = microcarrier-based bioreactor.
In addition to CoG, further considerations when selecting a technology for adherent cell expansion include time for process development. For example, implementing a straightforward technology such as multiplate bioreactors can take up to 12 months. However, when companies use a more advanced technology (e.g., microcarriers), process development can take six months to two years. In addition to process development costs and time, the capital investment required for different technologies is an important consideration. Figure 8 shows the relationship between the initial capital investment required and the manufacturing CoG for commercial-scale manufacture of adherent allo-CTs across multiple technologies and two annual-demand scenarios.
Technology selection is complicated further by the fact that process development can be significantly different for the same technology, depending on final desired lot size. For example, multilayer technologies enable fast-track development from low to medium lot size. But the capital investment requirement for large lot sizes becomes more substantial because it would require additional development and equipment to allow for adequate automation.
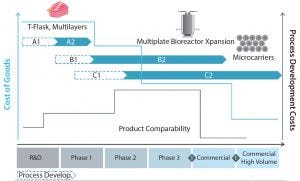
Figure 9: The path of both manufacturing cost of goods (blue line) and process development costs (grey line) in route to industrialization; arrows A1, A2, B1, B2, C1, and C2 represent the different technologies available for the large-scale manufacture of allogeneic adherent cells (25).
Figure 9 shows that with the clincal progression of a product, larger quantities of cells are required. That results in an increase in development costs (grey line), which begin to recede as commercial scale production is reached. As the volume of cells being manufactured increases, manufacturing CoG/million cells decreases (blue line) based on achieving greater economies of scale.
Summary and Outlook
Significant advances in cell therapies have been reported over the past decade with an increasing number of products approaching commercialization. Early planning is crucial for successful commercialization of allo-CT products. It includes process economics analyses to provide an understanding of a product’s economics, including cost drivers.
Other key factors can influence the ability of a novel CT product to succeed during commercialization:
Process development resource and time considerations
Reimbursement
Market potential and market certainty
Biological features of a product
Process robustness
Final product form
Technology limitations
Facility configuration (centralized or decentralized).
Determining the optimal manufacturing strategy early on will facilitate commercial success through identification of resource availability, technology gaps, and possible risks. Manufacturing technology selection greatly depends on the manufacturing scale selected, the cost-effectiveness of the different technologies at the selected scale, and the development effort required for commercial-scale manufacture.
Although traditional systems such as multilayer stacks are more commonly used at small scales, they are open and lack automation and capacity, making them prone to operator-related error. Thus, they are unsuited for commercial-scale expansion of CT products.
Single-use bioreactors substantially reduce overall costs and simplify QA and QC functions because they decrease the number of operations required and have higher capacity. New innovative bioreactors (e.g., the Xpansion multiplate bioreactor) can be operated as fully closed systems and provide a cost-effective means of cell expansion at mid-scale lots sizes of ≤100 billion cells.
Implementing a stirred-tank bioreactor system with microcarriers allows for much larger processing scales to be achieved within relatively small footprints, significantly reducing the costs of large-scale, adherent-cell manufacture. Those advantages must be balanced against the extra development effort that may be required (29).
Currently, the ultimate bottleneck for large-scale production of allo-CT products is the restricted capacity of DSP technologies. Increased focus on large-scale DSP technologies will help fill this gap particularly for high-dose, high-demand scenarios.
References
1 Mason C, Dunnill P. Quantities of Cells Used for Regenerative Medicine and Some Implications for Clinicians and Bioprocessors. Regen. Med. 4(2) 2009: 153–157; doi: 10.2217/17460751.4.2.153.
2 Ratcliffe E, Thomas R, Williams D. Current Understanding and Challenges in Bioprocessing of Stem Cell-Based Therapies for Regenerative Medicine. Br. Med. Bull. 100(1) 2011: 137–155; doi: 10.1093/bmb/ ldr037.
3 Wei X, et al. Mesenchymal Stem Cells: A New Trend for CT. Acta Pharmacol. Sinica 34(6) 2013: 747–754; doi: 10.1038/aps.2013.50.
4 Chen A, Reuveny S, Oh S. Application of Human Mesenchymal and Pluripotent Stem Cell Microcarrier Cultures in Cellular Therapy: Achievements and Future Direction. Biotechnol. Adv. 31(7) 2013: 1032–1046; doi: 10.1016/j.biotechadv.2013.03.006.
5 Simaria A, et al. Allogeneic CT Bioprocess Economics and Optimization: Single-Use Cell Expansion Technologies. Biotechnol. Bioeng. 111(1) 2013: 69–83; doi: 10.1002/bit.25008.
6 Mount N, et al. Cell-Based Therapy Technology Classifications and Translational Challenges. Phil. Trans. R. Soc. B 370(1680) 2015: 20150017; doi: 10.1098/rstb.2015.0017.
7 Kawada H, et al. Nonhematopoietic Mesenchymal Stem Cells Can Be Mobilized and Differentiate into Cardiomyocytes After Myocardial Infarction. Blood 104(12) 2004: 3581–3587; doi: 10.1182/blood-2004-04-1488.
8 Kelly M, et al. TNF Receptor 2, Not TNF Receptor 1, Enhances Mesenchymal Stem Cell-Mediated Cardiac Protection Following Acute Ischemia. Shock 33(6) 2010, 602–607; doi: 10.1097/shk.0b013e3181cc0913.
9 Malik N. Allogeneic Versus Autologous Stem-CT. BioPharm Int. 2012.
10 Heathman T, et al. The Translation of Cell-Based Therapies: Clinical Landscape and Manufacturing Challenges. Regen. Med. 10(1) 2015: 49–64; doi: 10.2217/rme.14.73.
11 Li M, Atkins H, Bubela T. The Global Landscape of Stem Cell Clinical Trials. Regen. Med. 9(1) 2014: 27–39; doi: 10.2217/rme.13.80.
12 Rosenblatt B. Meeting Regulatory Challenges for Cell-Based Therapies: Contrasting Novel and Familiar Biologics. BioProcess Intl. 10(3) 2012: S8–S11.
13 Bovy T, Fairbank A, Farid SS. Designing the Most Cost-Effective Manufacturing Strategy for Allogeneic CellBased Therapies. BioProcess Intl. 13(8) 2015: S36–S43.
14 Smith D. Assessing Commercial Opportunities for Autologous and Allogeneic Cell-Based Products. Regen. Med. 7(5) 2012: 721–732. doi: 10.2217/rme.12.40.
15 James D. Small Batch-Size Production: Addressing the Commercial Challenge. BioProcess Int. 10(3) 2012: S30-S35.
16 Hassan S, et al. Allogeneic CT Bioprocess Economics and Optimization: Downstream Processing Decisions. Regen. Med. 10(5) 2015: 591–609; doi: 10.2217/rme.15.29.
17 Raviv L, Karnieli O. Cell Therapies: The Challenges and Possible Solutions for Transferring CT from the Bench to the Industry. Drug Dev. Del. March 2014.
18 Prieels J, et al. Mastering Industrialization of CT Products: An Opportunity for Dedicated CMOs. BioProcess Int. 10(3) 2012: S12–S15.
19 Jones S, Mckee S, Levine H. Emerging Challenges in CT Manufacturing: Solutions from Monoclonal Antibodies. BioProcess Intl. 10(3) 2012: S4–S7.
20 Pereira Chilima T, et al. Impact of Allogeneic Stem Cell Manufacturing Decisions on Cost of Goods and Process Robustness. ECI Scale-Up and Manufacturing of Cell-Based Therapies IV, San Diego, CA, 2015.
21 Lapinskas E. Overcoming the Challenges of Cell-Based BioProcessing. Pharm. Technol. 2010.
22 Hass R, et al. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Comm. Signal. 9(1) 2011: 12; doi: 10.1186/1478-811x-9-12.
23 Rowley J, et al. Meeting Lot-Size Challenges of Manufacturing Adherent Cells for Therapy. BioProcess Int. 10(3) 2012; S1622.
24 Szczypka M, et al. Single-Use Bioreactors and Microcarriers: Scalable Technology for Cell-Based Therapies. BioProcess Intl. 12(3) 2014: 54–63.
25 Castillo J. Industrialization of Stem Cell Processes: How to Identify the Right Strategy? ISCT, Paris, 26 April 2014.
26 Lopez F, et al. A Quality Risk Management Model Approach for CT Manufacturing. Risk Analysis 30(12) 2010: 1857–1871. doi: 10.1111/j.1539-6924.2010.01465.x.
27 Gregory C, Prockop D. Chapter 9: Fundamentals of Culture and Characterization of Mesenchymal Stem/Progenitor Cells (MSCs) from Bone Marrow Stroma. Culture of Human Stem Cells. John Wiley & Sons: New York, NY, 2007; 207–232; doi: 10.1002/9780470167526.ch9.
28 Pattasseril J, et al. Downstream Technology Landscape for Large-Scale Therapeutic Cell Processing. BioProcess Intl. 11(3) 2013: S38–S47.
29 Hassan S, et al. Process Change Evaluation Framework for Allogeneic Cell Therapies: Impact on Drug Development, Manufacturing, and Commercialisation. Regen. Med. 2016: (in press).
Tania D. Pereira Chilima is an engineering doctorate researcher at University College London’s department of biochemical engineering working in collaboration with Pall Life Sciences. Thierry Bovy is global product manager of Xpansion multiplayer bioreactor systems at Pall Life Sciences. Corresponding author Suzanne Farid is a professor in bioprocess systems engineering at University College London’s department of biochemical engineering; [email protected].
You May Also Like






