Innovative Downstream Purification Solutions for Viral Vectors: Enabling Platform Approaches to Advance Gene TherapiesInnovative Downstream Purification Solutions for Viral Vectors: Enabling Platform Approaches to Advance Gene Therapies

Figure 1: Affinity chromatography principle: target-specific ligands are chemically immobilized or “coupled” to a solid chromatographic support. The complex mixture that contains the target molecule with impurities is loaded over the affinity column, and the target molecule that has specific binding affinity to the ligands on the resin will bind. Impurities are washed away, and the bound molecule is eluted from the column, resulting in its purification from the original feedstock.
Over the past decade, gene therapy applications and their importance in the biopharmaceutical industry have been increasing. Gene therapies promise versatile treatment options that could revolutionize and transform medicine. As treatment modalities, they offer the possibility of long-term and potentially curative benefits to patients with genetic or acquired diseases. Gene therapies are designed to treat disease by delivering genetic material that encodes a protein with a therapeutic effect into a patient’s cells. It can be used to replace a missing or faulty gene or correct the effects of a mutant gene, for example, with gene editing. Genetic material can be delivered to a patient’s cells either in vivo or ex vivo.
Although a number of molecular vectors and techniques are available for delivering therapeutic genetic material to target cells, it is most frequently achieved by using viruses that can be administered in vivo to patients either locally or systemically. Several types of viruses (e.g., retrovirus, lentivirus, adenovirus, and herpes simplex virus) can be used as delivery vehicles to infect and transfer a functional gene into patient’s cells. But vectors centered on the nonpathogenic adeno-associated virus (AAV) have emerged as the vector of choice for many therapies. AAV vectors can infect dividing and nondividing cells and mediate long-term tissue-specific gene expression, and they have a low immunogenicity. Twelve distinct subtypes of AAV have been identified, each varying in its tissue tropism (1).
A number of clinical studies involving recombinant AAV-based vectors have reported excellent clinical outcomes. And the clinical applications of these vectors has expanded to now include monogenic diseases, HIV and hepatitis infections, age-related macular degeneration, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, arthritis, epilepsy, and pain (3). In 2012, the first AAV-based medicine, Uniqure’s Glybera (alipogene tiparvovec) was approved by the European Medicines Agency as a treatment for patients diagnosed with the rare disease lipoprotein lipase deficiency. Since then, a recent survey of gene therapy products in clinical development worldwide identified a total of 213 products currently in development (2).
Although the biopharmaceutical industry is primed to purify viral vectors on a scale suitable for clinical supply or orphan indications, it is still working toward generating industrialized platform technologies that will maximize productivity and enable efficient, simple, and inexpensive purification of biologically active viral vectors for large-scale commercial manufacturing.
Here we outline key characteristics of POROS CaptureSelect AAV8 and POROS CaptureSelect AAV9 affinity chromatography resins — two products developed by Thermo Fisher Scientific’s BioProduction Purification Solutions Team in collaboration with industry colleagues — to purify AAV subtype 8 (AAV8) and subtype 9 (AAV9). They combine the unique properties of the CaptureSelect affinity technology platform with the large-pore, high-throughput features of the POROS perfusion chromatography resin to deliver a resin specifically designed for clinical-to-commercial scale affinity purification of AAV8 and AAV9 viral vectors. That combination enables a more efficient and higher yielding purification process than traditional methods. These resins provide an industrialized purification platform for efficient scale up and commercial manufacturing of gene therapies to ultimately meet market demands.
CaptureSelect Technology and Affinity Chromatography
CaptureSelect technology is used to produce high-binding ligands for affinity chromatography. This form of chromatography is proving to be an essential purification platform for many biotherapeutic molecules (including viral vectors) because it enables a high-purity and high-productivity process with fewer purification steps than traditional processes while offering process consistency and scalability (Figure 1).
The technology is based on the variable domain of Camelid heavy-chain-only antibodies (VHH), which as single antibody domains provide full functionality in antigen-specific recognition and high-affinity binding. Because of their compact structure, these domains are very robust and can withstand the various conditions as typically run in chromatography. During ligand discovery, the final ligand that meets all predefined requirements is selected and produced in an animal-origin-free (AOF) system in Saccharomyces cerevisae at any scale.
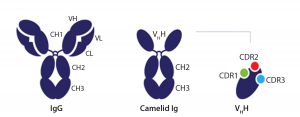
Figure 2: Comparison of a conventional antibody and a heavy-chain–only antibody as found in Camelids, which have the variable domain (VHH) (3)
Figure 2 shows the difference between a conventional antibody and a heavy-chain–only antibody, as found in Camelids that have the variable domain (VHH) (3).
CaptureSelect technology has been validated in numerous commercial biotherapeutic downstream purification processes, including blood coagulation factors, hormones, and antibody-derived therapeutics (4–6). And it was used to purify the first regulatory-approved AAV1-based gene therapy product in Europe. In this case, a VHH ligand against a specific AAV subtype was identified and developed to enable a one-step affinity chromatography procedure. The number of process steps decreased from the typical four or five to just one or two steps, with a related reduction in cleanroom time for the recovery process from weeks to days. In this case, step reduction improved product recovery from 30% to >60% (7).
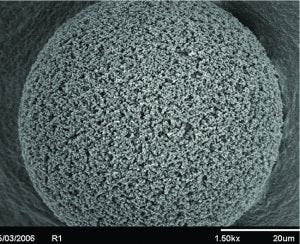
Figure 3: Scanning electron microscope (SEM) of a POROS bead showing large pore structures. The throughpores, in addition to the 50 µm bead size, increases capacity for viral vectors and other large biomolecules.
POROS and POROS CaptureSelect AAV8 and AAV9 Affinity Resins
POROS chromatography resins are rigid 50-mm polymeric beads (Figure 3), characterized by the addition of large “throughpores.” Their large pore structure make these resins ideally suited for the capture of large biomolecules such as viral vectors. When paired with the CaptureSelect technology, the resins enable a high purity and scaleable affinity-purification platform.
POROS CaptureSelect AAV8 and AAV9 affinity resins contain an immobilized ligand that specifically binds either AAV8 or AAV9 subtypes, respectively. The large-pore resin backbone consists of crosslinked poly(styrene divinylbenzene) and is coated with a cross-linked polyhydroxylated polymer. This coating is further derivatized with the CaptureSelect AAV8 and AAV9 affinity ligands.
Key Features |
|---|
POROS CaptureSelect AAV8 and AAV9 resins have several key features for AAV8 and AAV9 vector manufacturing from clinical to commercial scale: |
Combining Technologies for Large-Scale Purification
By contrast with many biotherapeutics such as proteins and nucleic acids, viruses such as AAV are quite large in terms of their weight and size and more complex in their composition — making them challenging to purify. It also is crucial that an efficient downstream purification process maintains the biological activity of an AAV vector while removing impurities and contaminants present in a feedstock that originates from host cells or culture media and to comply with strict regulatory guidelines. That is also critical to ensuring stable and effective intercellular transgene expression and to preventing immunological and biological responses and transmission of infectious diseases.
Current purification methods for AAV8 and AAV9 vector manufacturing consist of multiple steps that can include cesium chloride (CsCl) density-gradient centrifugation, iodixenol gradients, and several chromatography steps (e.g., ion-exchange chromatography, hydrophobic interaction, and heparin or IMAC affinity) and a concentration procedure. In addition to a lengthy cleanroom residence time, such a process can lead to long process development lead times, a more expensive process, and cumulative yield losses. This process also suffers from poor scalability, thereby limiting the commercial feasibility of any promising viral vector.
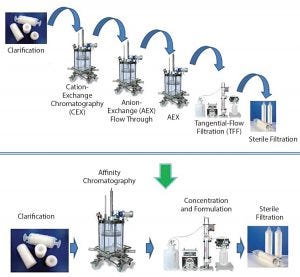
Figure 4: A paradigm shift in viral vector purification using the POROS CaptureSelect technology; standard purification processes require multiple chromatographic, filtration, and capture steps, which results in low yields and long process development timelines. POROS CaptureSelect technology obtains high purity and yield in a single step, which simplifies processes, shortens development timelines, and increases speed of therapeutics to market.
By contrast, combining POROS and CaptureSelect technologies has enabled a paradigm shift in AAV8 and AAV9 vector purification (Figure 4). Use of this combination frequently requires only a single capture step and then a concentration step, significantly simplifying a purification process. Fewer unit operations means higher product yield obtained, thereby reducing cost of goods (CoG), which ultimately enables speed to market.
The large pore structure of POROS CaptureSelect AAV8 and AAV9 affinity resins makes them suited for purifying large, complex AAV vectors, supporting chromatographic separations at considerably faster flow rates than conventional liquid-chromatography separations while maintaining high dynamic binding capacity. In addition, the 50-μm particle size provides superior resolution for unprecedented single-step impurity clearance that is independent of scale and flow rate. These affinity resins address the high-selectivity and high-capacity requirements for purifying viral vectors, exhibiting capacities of >1014 viral genome (vg)/mL (Table 1).

Table 1: Summary of POROS CaptureSelect AAV8 and AAV9 affinity resins properties; selectivity, binding capacity, and pH stability
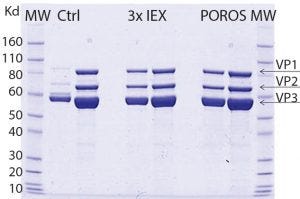
Figure 5: SDS-PAGE comparing purity of AAV9 viral vector, purified by two downstream processing methods; one is using three ionexchange (IEX) steps and the other with POROS CaptureSelect AAV9 resin as a one-step capture process. Data show that the purity profile of viral vector AAV9 is equivalent when comparing both downstream processing approaches. The gel also reveals similar purity, and the capsid viral protein (VP) topology for viral vector AAV9 is confirmed showing the bands corresponding to the viral structural proteins VP1, VP2, and VP3.
In a collaborative study with Généthon (a member of the Roche group) the purification of an AAV9 viral vectors using multiple ion-exchange steps was compared with POROS CaptureSelect AAV9 resin. Although equivalent AAV9 purity was obtained using both methods (Figure 5), using POROS CaptureSelect AAV9 resin reduced processing steps and increased yield.
For example, when using POROS CaptureSelect AAV9 affinity resin in the capture step during vector purification, a satisfactory vector recovery of ≥70% is obtained. Figure 6 shows that viral vector recovery is reproducible at different scales as process scale-up occurs 20-fold.
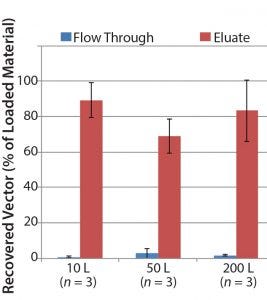
Figure 6: AAV9 vector recovery data at three different scales; three different AAV9 batches were produced at each scale to assess process reproducibility and to show scalability.
Généthon also noted that viral-vector purification processes are simplified when using POROS CaptureSelect AAV8 and AAV9 affinity resins, increasing product yield from 20% to 60% and reducing cost by a factor of six over alternative methods. This result is crucial because the industry is focusing on developing industrial capabilities to produce viral vectors in large amounts to meet clinical and future market demands.
POROS CaptureSelect AAV8 and AAV9 affinity resins are currently used to produce vectors supporting multiple clinical-phase programs and to design platform processes and large-scale production processes for vector purification. Through Thermo Fisher Scientific’s customization program, the company’s protein purification experts also are working directly with customers to develop additional affinity resin products to develop purification solutions for alternative gene and gene-modified cell therapy delivery vectors.
Valuable Platform Technologies
Gene therapy shows great potential in the treatment of several diseases. The industry is establishing efficient commercial manufacturing capabilities for gene therapies to meet market needs, and affinity chromatography is proving to be a valuable platform technology that increases process productivity.
POROS CaptureSelect AAV8 and AAV9 affinity resins are unique high-performing technologies designed specifically for AAV8 and AAV9 viral vectors. They offer high selectivity and capacity, resulting in a fast, robust process that generates high viral-vector product yield and purity, from crude material in a single-step purification process. Using them reduces the number of purification steps and offers scalability and processing consistency in the purification of viral vectors AAV8 and AAV9 for producing clinical-grade gene therapy molecules.
Acknowledgments
We thank Robert Kotin (NIH) for his contribution in helping to develop the AAV 9 ligand, now part of the POROS CaptureSelect AAV9 affinity matrix. We also thank our collaborator Généthon for providing us with data on its AAV9 purification process.
References
1 Vance MA, Mitchell A, Samulski RJ. AAV Biology, Infectivity and Therapeutic Use from Bench to Clinic. INTECH 2015; doi: 10.5772/61988.
2 Advances in Gene Therapy, Technology and Market Trends. Frost & Sullivan. September 2015.
3 Harmsen MM, De Haard HJ. Properties, Production, and Applications of Camelid Single-Domain Antibody Fragments. Appl. Microbiol. Biotechnol. 77, 2007: 13–22.
4 Winge S, et al. Development, Upscaling and Validation of the Purification Process for Human rhFVIII (Nuwiq): A New Generation Recombinant Factor VIII Produced in a Human Cell Line. Protein Expr. Purif. 115, 2015: 165–175.
5 McCue J, et al. Manufacturing Process Used to Produce Long-Acting Recombinant Factor VIII Fc Fusion Protein. Biologicals 43, 2015: 213–219.
6 Detmers F, Mueller F, Rohde J. Increasing Purity and Yield in Biosimilar Production: Taking Protein Purification to the Next Level. BioProcess Int. 11(6) 2013: S36–S40.
7 Smith RH, Levy JR, Kotin RM. A Simplified Baculovirus-AAV Expression Vector System Coupled with One-Step Affinity Purification Yields High-Titer rAAV Stocks from Insect Cells. Mol. Ther. 17(11), 2009: 1888–1896.
Orjana Terova is global product manager for POROS chromatography products, Bioproduction at Thermo Fisher Scientific (Bedford MA 01730). Shelly Parra is senior manager, global field applications at Thermo Fisher Scientific. Corresponding author Remko Clasen is senior market development manager, EMEA bioproduction, at Thermo Fisher Scientific; J.H. Oortweg 21, Leiden, 2333 CH, The Netherlands; [email protected]. Pim Hermans is director of ligand discovery, Thermo Fisher Scientific. CaptureSelect and POROS are registered trademarks of ThermoFisher Scientific. Stephen Soltys is field application scientist at ThermoFisher Scientific.
You May Also Like






