Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell TherapiesManufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies
October 17, 2016
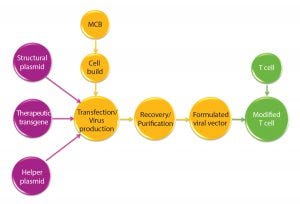
Figure 1: Manufacturing overview for viral vectors and modified T cells; MCB = master cell bank
The concept of gene therapy is far from new, with initial studies performed over 20 years ago (1). However, in the past few years an explosion of interest in this area has gone beyond initial regenerative approaches using viral vectors. Interest is now moving increasingly into potential use of T cells modified using recombinant viral vectors for immunotherapy applications. Such therapies are based on using either adenoassociated virus (AAV) or lentivirus (1), both vectors being frequently generated through transient expression routes based on plasmid DNA as a starting material.
The viral vectors are generated using two, three, or four plasmid constructs for each vector with two or three structural/helper genes and a single therapeutic transgene. These are then used to generate the viral vector of choice (Figure 1).
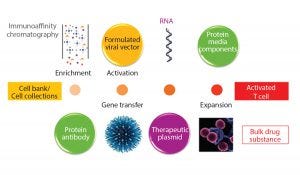
Figure 2: Starting material for modified T-cell production
It is widely recognized that manufacturing modified T cells at the scales needed for large patient numbers raises many challenges from a scientific, technical, and cost-ofgoods perspective. Processes for these products take biomanufacturing to a new level of complexity — exemplified by the number of biological entities required to be produced, themselves manufactured through recombinant routes (Figure 2).
With improving levels of clinical success and the progression of products into late-phase clinical studies, the industry will see an inevitable need for increased amounts of plasmid DNA to be made at larger manufacturing scales. Alongside that, it is reasonable to expect greater regulatory scrutiny of manufacturing processes as more patients are exposed to these types of products. Looking further forward, as these types of clinical approaches continue to show positive clinical outputs, drug developers will need to increasingly “plan for success” and develop long-term manufacturing strategies that can be carried through into later clinical phases and in-market supply.
A number of companies are recognizing a need for significant changes in manufacturing approaches for producing the cell therapies themselves, and to a large degree for viral vector manufacturing processes to meet the requirements for late-phase clinical trials and in-market demands for successful products. Technical solutions are needed to enable these changes.
I do not doubt that the need to manufacture greater amounts of plasmid DNA will increase. To meet rising demand, production platforms will need to be scaled up significantly. It may be possible to meet the demands for niche therapies of <10,000 patients with small-scale production platforms making <10g/batch. But a reasonable prediction is that 100–1,000 g/year will be required for each plasmid vector for a marketed product.
The implication of this increased need for material is that road maps must be created to guide process changes for manufacturing viral vectors and plasmid DNA. Those guides also need to demonstrate comparability with regards to safety and functionality.
Taking this a stage further, as products progress through clinical phases, their developers must build robust supply chains. Good manufacturing practice (GMP) guidelines suggest establishing back-up suppliers to make critical materials for late-phase and in-market products (2). If this is the case for plasmid DNA, then it may well involve not only sourcing materials from different suppliers and facilities, but also incorporating different manufacturing processes.
Glossary |
|---|
Modified T Cells: reprogrammed T lymphocytes that target cancer-specific abnormalities so that malignant cells can be targeted and attacked throughout the body; www.cityofhope.org/blog/adoptive-t-cell-fight-cancer. |
Adeno Associated Virus (AAV): the parvovirus family of small viruses with a genome of single stranded DNA; they can insert genetic material at a specific site on chromosome 19 with near 100% certainty; www.genetherapynet.com/viral-vector/adeno-associated-viruses. html. |
Lentivirus: Retroviruses with long incubation periods; they can cause chronic, progressive, often fatal diseases in humans and other animals; www.genetherapynet.com/viral-vector/lentiviruses.html |
MCB: master cell bank |
IEX and HIC: ion-exchange chromatography and hydrophobic-interaction chromatography |
HPLC and CE: high pressure liquid chromatography and capillary electrophoresis |
Regulatory Perspective of Plasmid DNA Production
The responsibility for supply-chain management clearly falls on drug developers and trial sponsors who are ultimately responsible for the quality of their products. Those groups are required to provide materials for early stage clinical evaluation and to establish a road map for supplying materials for late-stage clinical studies and in-market needs.
When we look at the approaches taken to supply plasmid for early stage clinical trials, it is clear that many companies are using high-quality plasmid DNA. The 2005 EMEA guideline on “Development and Manufacture of Lentiviral Vectors” (3) cites the need for using high-quality DNA in production of lentiviral vectors and a requirement to provide full gene sequences for each plasmid and transfer vector package. However, no real guidelines exist to describe quality systems or manufacturing approaches for these high-quality products. Many of them do not fit within the principles of GMP. Suppliers including my company offer services in this area, each with its own approach to levels of traceability of raw materials and consumables, environmental control, and final-product testing including sequence integrity.
From FDA Guidelines 2007 |
|---|
% Supercoiled plasmid > 80% |
Endotoxin < 40 Eu/mg |
Host DNA < 1% |
Host protein < 1% |
High-quality plasmids are currently used only in early stage “proof of principle” studies on very small patient numbers (<20). But how the approach will progress with successful products and increased patient numbers has not been widely discussed. The issue is complicated by references to “quality, nonclinical, and clinical aspects of gene therapy medicinal products” (4), among other products mentioned, for which plasmid DNA is deemed to be a GMP-level “starting material” and is required to be produced under the “principles of GMP” from the cell bank onward. However, as with the terminology of “high quality,” the phrase “principles of GMP” is open to differing interpretations, as is the alignment of the two requirements. It is clear that some larger companies have taken the approach that GMP standards are applied throughout, even for the supply of phase 1–2a clinical materials, with other product development groups taking a much less stringent approach. It is also apparent that these more recent guidelines push further toward application of ICH guidelines for not only gene therapy products, but also ex vivo viral vector modalities.
Classifying plasmids as a starting material is not surprising given that they provide the starting genetic code for protein elements that bring about a desired therapeutic benefit. Assessing the impact of coding errors is likely to be challenging in terms of patient safety and therapeutic impact. For starting materials, the MHRA 2015 guidelines suggest that a “level of supplier supervision should be proportionate to the risks posed by the individual material taking account of their source, manufacturing process, supply chain complexity and the final use to which the material is put in the medicinal product” (5). Those guidelines may provide a useful starting point when determining quality approaches to plasmid production.
Applying increasing levels of GMP stringency based on clinical phase is not unprecedented. PDA Technical Report 56, “Application of Phase Appropriate Quality Systems and CGMP to the Development of Therapeutic Protein Drug Substance,” provides guidance for more conventional biologics and for drug developers (2). That document cites four specific areas to be assessed: quality systems, facilities, equipment, and materials and laboratory, with the main differentiations made between approaches at phase 2 and those at phases 2 and 3. Because many cell and gene therapy products are unlikely to enter classical phase 1 safety studies, assessments need to be made based on patient numbers and associated risks, including those incurred by compassionate use.
The guidelines also recognize various organizational structures and capabilities with different levels of compliance for early phase studies:
Large pharmaceutical/biotechnology companies that are likely to take products through to in-market supply
Start-up organizations that will develop products to phase 1–2 and then transfer them to, or partner with larger organizations
Universities and grant-funded bodies performing small trials for scientific publications.
To date, many gene therapy studies have been sponsored by university and hospital-based groups, but this is rapidly changing. Lines are blurring as large pharmaceutical and well-funded start-up companies work alongside academic groups in early clinical evaluations. The approach to quality systems needs to be risk based and requires an understanding of the nature of the starting material, the manufacturing processes behind it, and the intended use of the product.
In the case of plasmid DNA, manufacturing is invariably outsourced to specialist manufacturers using in-house platform processes. In such circumstances, limited (if any) development studies of the specific plasmid vector production are performed up front. Instead, sponsors rely on the knowledge and understanding the manufacturer has of its own production platform rather than knowledge gained for a specific plasmid product. It is apparent that, to date, the selection of suppliers is based as much on cost and availability as it is on risk.
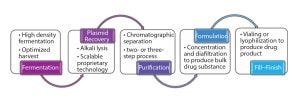
Figure 3: Outline of plasmid DNA production process
Manufacturing Processes
From a technical perspective, manufacturing processes used for the production of clinical-grade plasmid DNA are fairly similar (Figure 3).
Plasmids and Cell Banks: Although regulators identify a cell bank as the starting point of a GMP envelope, in terms of absolute starting points for plasmid DNA, an initial plasmid construct is used to generate manufacturing cell banks. The identity, integrity, and stability of the plasmid DNA is critical to the future of a product and provide the foundation of the process used to manufacture an intended viral vector and achieve the intended therapeutic benefit.
Usually the plasmid backbone is the starting material provided to DNA producers. Those plasmids themselves are generated in-house by the drug developers who transform them into K12-based E. coli production strains. From the initial work, clones are selected before fairly basic assessments and ahead of cell-bank generation. One requirement of the ICH Q5D guidelines (11) on cell banks is to demonstrate plasmid stability — with regard to plasmid loss, segregational loss, and rearrangement — for extended generations under process conditions. Such events are not unheard of with viral vectors and may require reengineering the plasmids.
When assessing plasmid constructs and cell banks specific to “principles of GMP,” you can find a number of FDA and EMEA guidelines on the requirements for their construction, manufacturing, and testing (6). Guidelines include recommendations on the use of antibiotic marker genes such as avoiding the use of ampicillin; concerns have been raised by both the FDA and EMEA expressing an overall preference for antibiotic-free systems (7–9). Although some developers choose not to generate fully tested GMP cell banks for early clinical phases, the implications of needing to change cell banks must be considered as do approaches for generating GMP cell banks to support later stage clinical trials using existing research cell banks (7).
Fermentation and Downstream Purification: Fermentation processes, tend to be fairly similar and usually run in fed-batch mode. They are likely to be antibiotic free and follow the industry practice of using chemically defined media, especially for later phase and large-scale processes. The cell paste generated from fermentation is harvested and the plasmid extracted from the cell paste by a process of alkaline lysis. This tends to be where process differences lie, with manufacturers using proprietary cell lysis technologies to extract plasmid DNA. Precisely how different approaches affect the quality and functionality of a product is difficult to assess and not reported in scientific literature.
Purification processes are usually a combination of ion-exchange (IEX) and hydrophobic-chromatography (HIC) steps, and in some cases include gel-permeation (size-exclusion) chromatography as well. These processes use IEX to remove the endotoxin and residual RNA, and HIC to remove host DNA and critically separate out open- and closed-circle plasmid forms. Processes also may include precipitation steps to remove host RNA levels.
Analytical Testing and Specifications: Currently most drug development companies base their specifications on the FDA 2007 guidelines for injectable DNA vaccines (1) (see the FDA Guidelines box) although the expectation for closed-circle DNA level is usually 90% supercoiled rather than 80% as cited. Although analytical procedures do vary, fairly standard approaches are taken with regard to measuring residuals. Different approaches are taken to the quantification of plasmid forms, with some groups favoring HPLC-based procedures and others preferring CE-based methods.
The one area that is not covered in those guidelines is measurement of potency and functionality. Although plasmid supercoiled levels often are seen as a surrogate measure of plasmid functionality and stability and of transfectability with regard to production of viral vectors, not much evidence consistently supports this link. It is therefore challenging to identify meaningful critical quality attributes for plasmids used for generating viral vectors. Clearly, this is going to be crucial going forward as manufacturing processes are developed to meet future potential needs. Similarly, we also need to identify those attributes that are less critical.
Future Potential Manufacturing Approaches
Understanding critical quality attributes is going to be increasingly important. The need for changes in plasmid production processes and scales increases as manufacturers seek to identify approaches that achieve the required manufacturing scales and process flexibilities while also achieving acceptable levels of cost of goods (CoG). For example, the current expectation is for material to be purified to the specifications for injectable products with regard to residual contaminants. This is contrary to the approach some companies are taking to lentivirus production for which only limited purification is performed to remove levels of host contaminants — justified by robust demonstration of clearance in the cell therapy product. Can similar approaches be taken for plasmid DNA to allow for simplification of purification processes? We also need to look at the design of manufacturing facilities. Do we need to produce these products in high-grade cleanrooms, or can much of the production be performed in lower classification suites relying on closed-system approaches, whether it be through fixed or single-use facilities?
In terms of quality systems, we may also want to look at aspects of process validation. If plasmids are generated using platform-based approaches, including cleaning validation and the risk-assessment regarding extractable and leachables for single-use systems, can we validate the platform for production of multiple rather than single products?
Looking further ahead, we consider alternative approaches to plasmid manufacturing. Synthetic manufacturing processes that are used to make smaller DNA fragments —such as those developed by Touchlight (www.touchlight.com) with its Doggybone DNA (dbDNA) platform — may be used to generate viral vectors. In very early stage development, they could potentially simplify manufacturing processes. This also suggests that such small DNA fragments might be regarded as chemical (rather than biological entities) that can be fully characterized.
Supply Chains
Supply-chain management will be increasingly critical to support the potential requirement for manufacturing two to four plasmids for each viral vector and for each therapy going into trials and for in-market products. Also, with nearly all plasmid DNA manufacturing being outsourced to specialist manufacturers and CMOs, it is going to be incumbent on drug developers to seek long-term solutions with such suppliers to plan for future market needs. This includes identifying appropriate risk-based manufacturing approaches and supporting both the development of and investment into required capability and manufacturing capacity. It also will require flexible approaches that are not necessarily fixed upon any single platform, especially for in-market products, to ensure supply integrity. The future may well hold alternative approaches to meeting plasmid requirements, allowing reduction in CoG.
Bringing Promises to Reality
We are in very interesting times with the increasing emergence of gene and cell therapy products. As companies seek to take them to market, manufacturing challenges are being identified and solutions sought. It is clear that we need to reawaken industry investment in plasmid production platforms, including novel approaches. Additionally, greater clarity is required for the quality systems in place to support and direct the investments and developments required to accelerate production of these life-changing new therapies.
References
1 Roots Analysis. Gene Therapy Market 2015–2025; www.rootsanalysis.com/reports/view_document/gene-therapy-market-2015-2025/85.html.
2 Eylath A, et al. PDA Technical Report No. 56: Application of Phase-Appropriate Quality Systems and CGMP to the Development of Therapeutic Protein Drug Substance; www.pdfbookstore.com.
3 European Medicines Agency, Committee for Medicinal Products for Human Use. Guideline on Development and Manufacture of Lentiviral Vectors (CHMP/BWP/2458/03); www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003984.pdf.
4 European Medicines Agency. Quality, Non-Clinical and Clinical Aspects of Gene Therapy Medicinal Products (Draft Guideline, EMA/CAT/80183/2014); www.ema.europa.eu/docs/en_GB/document_library/Scientific_ guideline/2015/05/WC500187020.pdf.
5 Medicines and Healthcare Products Regulatory Agency. Rules and Guidance for Pharmaceutical Manufacturers and Distributors (Orange Guide, 2015); http://www.pharmpress.com/product/9780857111715/orangeguide.
6 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Quality of Biotechnological Products: Analysis of the Expression Construct in Cells Used for Production of R-DNA Derived Protein Products (CPMP/ICH/139/95), November 1995; www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q5B/Step4/Q5B_Guideline.pdf.
7 US Food and Drug Administration. Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human
Somatic Cell Therapy Investigational New Drug Applications (INDs), April 2008; www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm092705.pdf.
8 US Food and Drug Administration, CBER. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy, March 1998; www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm081670.pdf.
9 European Medicines Agency, Committee for Proprietary Medicinal Products. Notes for Guidance on the Quality, Preclinical and Clinical Aspects of Gene Transfer Medicinal Products, April 2001(CPMP/BWP/3088/99); www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003987.pdf.
10 US Food and Drug Administration. Guidance for Industry: Considerations for Plasmid DNA Vaccines for Infectious Disease Indications, November 2007; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500003987.pdf.
11 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Q5D: Quality of Biotechnological Products: Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/Biological Products (CPMP/ICH/294/95), March 1998; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003280.pdf.
Supporting Documents
European Medicines Agency. Guideline on Quality, Non-Clinical and Clinical Aspects of Medicinal Products Containing Genetically Modified Cells (CHMP/GTWP/671639/2008), April 2012; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/05/WC500126836.pdf.
European Medicines Agency. Reflection Paper on Quality, Non-Clinical and Clinical Issues Relating Specifically to Recombinant Adeno-Associated Viral Vectors (CHMP/GTWP/587488/07), June 2010; www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/07/WC500094345.pdf.
Tony Hitchcock is technical director, Cobra Biologics, Stephenson Building, Keele Science Park, Keele ST5 5SP UK; [email protected].
You May Also Like






