- Chromatography
- Continuous Bioprocessing
- Sponsored Content
Continuous Chromatography Is Now Possible for Clinical ManufacturingContinuous Chromatography Is Now Possible for Clinical Manufacturing
Sponsored by Cytiva
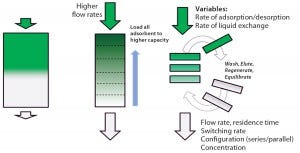
Figure 1: Principles of simulated moving bed technology
Intensified and integrated bioprocess technologies are creating a paradigm shift toward more efficient, higher flexibility facilities for biopharmaceutical manufacturing. Continuous technologies that are designed as single-use systems help to greatly facilitate process intensification, delivering further efficiencies with reduced set-up times and elimination of the need for cleaning and cleaning validation.
Chromatography is often considered to be a challenging bioprocess step, which has caused great interest in a simplified, safer solution. Continuous multicolumn chromatography using a single-use flow path is an economically viable, disposable chromatographic solution for even the most demanding and costly chromatographic steps in a manufacturing process.
The Cadence BioSMB PD platform from Pall Life Sciences is the first disposable flow path, continuous multicolumn chromatography solution that is scalable from a process development (PD) laboratory to GMP manufacturing and is designed for easy integration with other unit operations to allow integrated continuous manufacturing.
Read the full text of this article in the PDF (Login required).
You May Also Like





