Reducing Clinical-Phase Manufacturing Costs: Collaborating for Savings without Compromising Quality or PerformanceReducing Clinical-Phase Manufacturing Costs: Collaborating for Savings without Compromising Quality or Performance

Photo 1: A 30-cm OPUS column containing CaptivA resin connected to an ÄKTA Ready skid (GE Healthcare) for a good manufacturing practice (GMP) operation
In downstream purification of monoclonal antibodies (MAbs), the single greatest contributor to manufacturing costs is the expensive capture step typically based on protein A affinity chromatography. Almost since its introduction to bioprocessing, efforts have been made to reduce the cost of this step.
Several alternative ligands have been promulgated as potential replacements for protein A, but they have proven difficult to adopt and scale up. Supplier companies have pushed for increases in capacity and economics, but those are always accompanied by a corresponding increase in purchase price. Although increased capacity, higher flow rates, and increased caustic and process stability all have their price, Repligen believes that in most cases at clinical-phase manufacturing, little or no economic benefit comes from the extended cleanability or capacity that such “state-of-the-art” capture resins provide.

Photo 2: A 30-cm Repligen OPUS column containing CaptivA resin connected to an ÄKTA Ready skid (GE Healthcare) during a wash cycle for a GMP manufacturing operation
Disposables (single-use technologies) have become standard at many of world’s leading biopharmaceutical companies. They offer faster product changeover than multiuse technologies can, with favorable economics and improved safety. As a supplier of protein A affinity resins, Repligen proposed that significant savings in clinical manufacturing could be made by using a protein A resin that balances price and performance. Combining that with prepacked chromatography column technology could help users realize significant savings without adversely affecting process performance or product quality. So Repligen teamed up with Goodwin Biotechnology, a biological contract development and manufacturing organization (CDMO) focused on producing biologics such as MAbs derived from cell culture, and a contract customer. Together, we demonstrate that prepacked protein A columns would work in a real manufacturing setting for different MAbs at both small and large scales — and that they could provide savings to contract clients and manufacturers themselves.
This collaborative work shows that adoption and thoughtful, phase-appropriate deployment of new technology can provide a winning alternative for MAb manufacturing without compromising product quality or production timelines. So this approach should offer significant economic benefits for any company involved in clinical biomanufacturing.
Implementation of New Technologies
Our studies show how collaboration between a provider of new technologies and a user of those technologies can benefit end customers (e.g., contract clients) and ultimately patients who can better access lower-cost biological therapies.
The single most important and widely used step in MAb purification is affinity purification with protein A chromatography. This technology provides a high level of purity in one easy step. Its primary downside is the high cost of protein A resins. Repligen sells CaptivA protein A affinity resins based on a recombinant protein A ligand coupled to an agarose-base bead matrix using multipoint-attachment chemistry. The result is a capture resin that performs similarly to higher priced, well-established, and commonly used resins. Data generated in laboratory testing show that protein A leaching, contaminant removal (of both host-cell proteins, HCPs, and DNA) as well as caustic and process stability are similar to those attributes of high-cost, established resins.
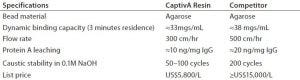
Table 1: Properties of CaptivA resin compared with a leading protein A affinity resin
Table 1 summarizes key comparative attributes of CaptivA resin and an alternative agarose-based protein A affinity resin. We believe that using this resin in the initial step of MAb purification could save about two-thirds of the usual cost for that step without compromising product quality. To determine whether this represents a viable alternative for its MAb purification operations, Goodwin Biotechnology conducted a comparative study of purification performances for both resins in multicycle purification of two MAb isotypes at both small- and large-scale manufacturing processes.
Comparative Studies

Table 2: Dynamic binding capacities (DBCs) for CaptivA resin and an alternative protein A affinity resin
The first study compared dynamic binding capacity (DBC) for both protein A affinity resins. Goodwin began with two columns: one packed with 1.5 mL of the alternative resin and one packed with 1.5 mL of CaptivA resin. Each column was loaded with a maximum of 65 mg MAb (a whole IgG1 type antibody) in clarified culture media with a residence time of six minutes for both columns. Maximum capacity was calculated at 10% of the monitored breakthrough. DBCs for both resins were essentially comparable in this process (Table 2).
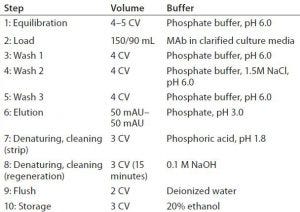
Table 3: Process-based cycling study
Cycling Studies Design: Next, Goodwin Biotechnology examined resin reusability. A multicycle study, based on a client’s antibody product, tested both CaptivA resin and the alternative protein A affinity resin (Table 3). Again, the analysts used two 1.5-mL columns packed with the respective resins. Three cycles were performed on the alternative resin column, and over 10 cycles were performed on the CaptivA column.
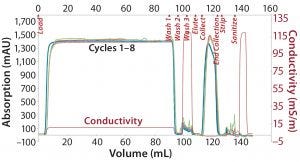
Figure 1: Overlay of CaptivA resin chromatograms
Note that after each cycle, the resins were regenerated with a very stringent cleaning using 0.1 M NaOH. Figure 1 overlays chromatograms from the CaptivA affinity runs, and Figure 2 overlays chromatographs from the alternative protein A affinity resin runs. Table 4 lists quality results for the eluted product. In terms of monomeric purity for this particular IgG1 molecule, all eluates contained 100% monomer. In a second study using a different IgG2a molecule, the monomer percentage was >99% for both resins (Figure 3).
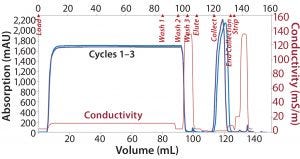
Figure 2: Overlaid chromatograms of alternative protein A affinity resin runs
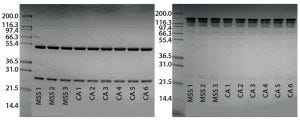
Figure 3: Reduced and nonreduced SDS-PAGE analysis of eluates from an alternative protein A affinity resin and CaptivA resin runs; (left) reduced SDS-PAGE, (right) nonreduced SDS=PAGE; MSS 1,2,3 = alternative protein A affinity resin cycles; CA1-6 = first six CaptivA resin cycles
Discussion of Results
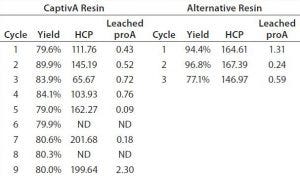
Table 4: Comparative results from cycling two protein A affinity resins
In this study, CaptivA resin presented with lower yields because of the pH 6 loading condition. Analysts did not optimize the process conditions because yields obtained were deemed acceptable for the application. Table 5 shows savings calculated from using CaptivA resin for different batch sizes.
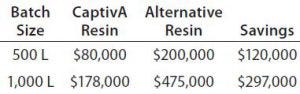
Table 5: Economic advantage provided by CaptivA resin in the MAb capture step
The comparative study of resin use and cycling (Table 4) shows that chromatographic performance and product quality are equivalent for both resins. DBC appears to be slightly lower for CaptivA resin with the IgG1 molecule studied. However, it is very close to that of the alternative resin, having no discernible effect on the amount required for a purification campaign. Both resins give similar yield and purity of eluted IgG1.
Streamlining Clinical and Commercial MAb Manufacturing with Prepacked Columns: A great benefit for biomanufacturers is using columns (such as those in Repligen’s OPUS line) that are prepacked with high-performance, low-cost protein A resin. This technology offers multiple advantages over traditional approaches:
Removing the need for column packing, sanitization, and qualification
Potentially reducing costs for protein A purification with a low-cost and high-performance resin
Reducing preparation times and demands on personnel resources in downstream process suites.
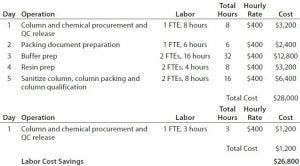
Table 6: Labor cost savings of a prepacked, single-use column compared with a conventional chromatography process (10 L)
We examined the hidden costs associated with packing and qualifying columns for manufacturing-scale process chromatography. Facility charge rates vary from $300/h to $500/h (fully burdened) for most good manufacturing practice (GMP) compliant biomanufacturing operations. Therefore, analysts chose a rate of $400/h for this exercise. As an example, the focus of this study is on the costs for packing a relatively small column with a 500-mL resin bed and a larger column with a 10-L resin bed.
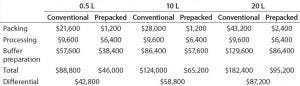
Table 7: Cost and savings summary comparison
Next, they examined operational costs associated with personnel in a GMP environment. And finally, they considered buffer preparation costs associated. Costs for packing and qualifying the column (scaled up to a 10-L resin bed) also were included. Personnel costs remain the same because the operational parameters remain similar. Buffer preparation costs become as outlined in Table 6. And Table 7 summarizes both the costs and savings that result.
Procurement costs for prepacked columns are higher than those for the individual components (e.g., columns and resins separately). But reducing the operational costs significantly outweighs that differential. Moreover, if a comparable low-cost substitute for a high-cost resin is available, then savings can be magnified substantially.
Review of Product Quality from Manufacturing Operations: Analysts ran purification campaigns for two different MAbs (an IgG1 and an IgG2a) to be used in clinical studies. Both campaigns involved highperformance, low-cost CaptivA protein A resin prepacked in 30-cm ID OPUS columns (Photos 1 and 2 on the top of this article) using identical buffer systems and process parameters.
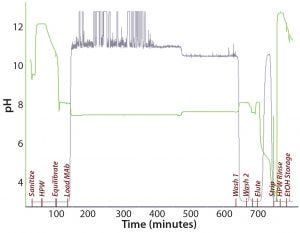
Figure 4: Chromatogram from purification of an IgG1 MAb on a 30 × 12 cm OPUS column packed with CaptivA resin
The prepacked columns had 12-cm bed heights packed with CaptivA resin. Clarified culture media from a 500-L bioreactor was loaded onto the column, which had been equilibrated with a phosphate buffer at pH 8.0, at a flow rate of 150 cm/h. Three wash steps were performed after the load (Figure 4), with a high-salt wash as the second step in that series. MAb elution was achieved by lowering the pH to 3.0. Following that, analysts held the elution pool at pH 3.5 for one hour to achieve virus inactivation.
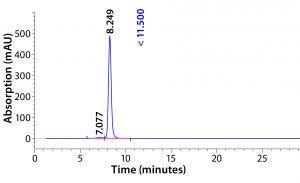
Figure 5: HPLC-SEC profile of CaptivA resin elution product
Then they adjusted pH to 5.0 for the subsequent cation-exchange purification step. Antibody quality was assessed following viral inactivation by testing purity with sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and size-exclusion high-performance liquid chromatographpy (SE-HPLC). Yield and concentration were determined by measuring optical density at 280 nm and an enzyme-linked immunosorbent assay (ELISA).
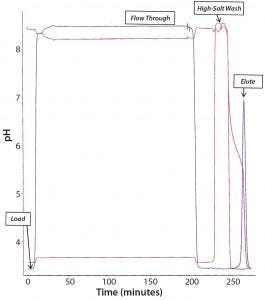
Figure 6: Chromatogram of the CaptivA resin capture step
The next steps of this purification process were cation-exchange chromatography followed by viral filtration and anion-exchange membrane chromatography, then finishing with diafiltration. Analysts tested the final MAb for purity using SDS-PAGE and SE-HPLC and measuring leached protein A and residual HCPs and DNA. Tables 8–11 summarize the results (see also Figures 5 and 6). They demonstrate that this MAb purification process is amenable to the use of prepacked chromatography solutions. Product quality remains very good with a low-cost, high-performance capture affinity resin.

Table 8: CaptivA resin elution concentration and yield for an IgG1 MAb
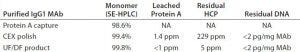
Table 9: Quality attributes of a purified IgG1 MAb with CaptivA resin in a prepacked OPUS column as the capture step
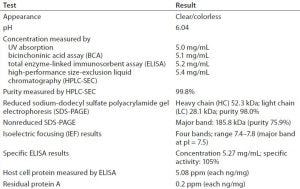
Table 10: Analysis of quality metrics for a purified IgG1 MAb

Table 11: CaptivA resin elution concentration and yield for an IgG2a MAb
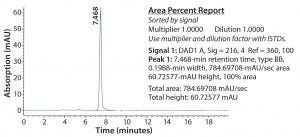
Figure 7: SEC-HPLC chromatogram from CaptivA resin elution of IgG2a product
Expanded Opportunity for Early Phase Clinical Manufacturing Cost Containment: Having seen the potential savings related to CaptivA resin, the CMO and its client wondered whether further savings could be achieved by bringing in ready-to-use, prepacked columns filled with that resin. In an effort to evaluate the potential for even more cost savings — and to add reliability from MAb process development to manufacturing — Repligen proposed OPUS prepacked chromatography columns with CaptivA resin. Goodwin Biotechnology and its client agreed to extend the economic assessment to that platform (Figure 7). The contract manufacturer is implementing the range of columns into its existing process flow plans.
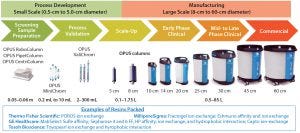
Figure 8: The range of OPUS prepacked columns for process development to manufacturing of biological products; from 1.2 cm to 45 cm in diameter, they can be packed at different bed heights with nearly any choice of chromatography media
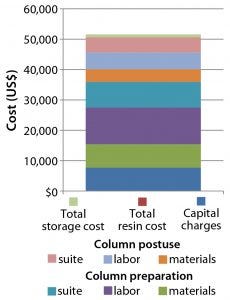
Figure 9: Cost of preparing a traditional 30-cm glass column ready for use in a GMP facility
In conjunction with Bioprocess Technology Consultants (BPTC), Repligen developed a predictive model for identifying available cost savings from “outsourcing” column packing. This model identifies each task required to bring a traditional glass column into a current good manufacturing practice (CGMP) facility. It takes into account labor time and rate for each task along with the amount of raw materials and solutions required and related sourcing costs. Each input is made available for review. Using the model, it is now possible to ascribe a dollar and labor-resource cost to an organization for preparing, packing, cleaning, and storing glass columns for reuse. If that cost is compared with the prepacked-column cost, the potential for savings can be seen immediately when implementing prepacked columns in place of traditional self-packed glass columns. Figure 9 shows typical costs associated with delivering a 30-cm diameter packed glass column ready for use in a GMP facility.
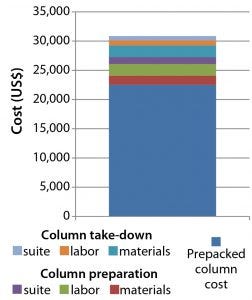
Figure 10: Cost of procuring and preparing a prepacked 30-cm OPUS column ready for use in a GMP facility
Figure 10, shows the cost for delivering a ready-to-use prepacked OPUS column of the same diameter and specifications — with a savings of 40% (US$21,000).
Combining those savings with that from using high-performance, low-cost CaptivA protein A resin yields dramatic potential economic implications. The cost for an organization to deliver a 30-cm ID, 12-cm bed-height glass column packed with an alternative protein A affinity resin ready for use in a GMP facility is about $280,000 (Figure 11). An equivalent 30-cm OPUS column packed with CaptivA resin would cost that same organization about $137,000 to implement into a GMP-ready process (Figure 12). That would allow a CMO to save its contract client >$140,000 for an early phase clinical manufacturing process.
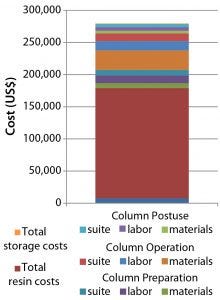
Figure 11: Cost of a 30-cm × 12-cm glass column self-packed with an alternative protein A affinity resin ready for use in a GMP facility
Save Time, Save Money
Working collaboratively, the supplier, contract manufacturer, and biopharmaceutical client have demonstrated that for two different MAbs in both small- and large-scale manufacturing processes, adopting a cost-effective protein A resin can significantly minimize cash burn for a startup company in the early phases of clinical development. CaptivA resin is functionally capable of replacing more expensive protein A resins without compromising product quality or process workflows. Combining that with adoption of prepacked chromatography columns brings even more pronounced client savings, exceeding $100,000 at the 30-cm scale.
Note that the contract manufacturer also expects to obtain benefits from implementing the prepacked OPUS chromatography platform. Improved profitability, organizational effectiveness, and ultimately customer satisfaction expect to be accrued through increases in efficiency that can be broadly characterized in three key areas: resource management, manufacturing timing, and facility turnaround.
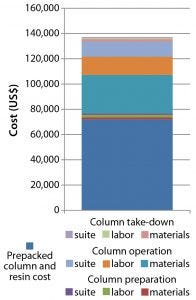
Figure 12: Cost of procuring a prepacked 30-cm × 12-cm OPUS column packed with CaptivA resin ready for use in a GMP facility
Resource Management: Biomanufacturers need to focus their highly skilled, highly compensated resources on value-added tasks other than column packing. Many companies report losing 10–25% of their packing time to repacks and outright pack failures. Such inefficiencies can be prevented by using prepacked columns instead.
Manufacturing Timing: If chromatography columns are not packed and ready to go for the start of a GMP downstream processing campaign, manufacturing slots can be lost and entire client processes delayed with the risk of a significant “knock on” effect. This adversely affects profitability and customer satisfaction. Purchasing prepacked columns ready for GMP processing helps biomanufacturers limit these risks.
Facility Turnaround Time: Count the number of days it takes in suite to pack and unpack three columns for a typical MAb process. Multiply that by a number of campaigns throughout the year, and consider what could be done with that time instead. Prepacked columns can shorten facility turnaround times, ultimately helping to make room for more campaigns in the same period.
A Savings Equation
Finally, note that savings could be multiplied by a factor of three: one for each process purification step that can be switched to a prepacked column in a typical three-step purification process. The resulting savings then would be multiplied again by the number of campaigns in time to achieve a full program savings:
Total Savings = C(P × S)
where C = number of campaigns, P = number of purification steps, and S = savings per step.
Dana C. Pentia is a senior application scientist, Arielle Fabiano was an R&D intern, James R. Peyser is senior director of bioprocess development, and corresponding author Steve Tingley ([email protected]) is vice president of sales and marketing at Repligen Corporation in Waltham, MA, www.repligen.com. Maryel Gonzalez-Perez is senior manager of assay development and quality control, Jack Vicalvi was manager of downstream process development, and corresponding author Muctarr Sesay, PhD, ([email protected]) is chief scientific officer at Goodwin Biotechnology in Plantation, FL, www.goodwinbio.com. CaptivA and OPUS are trademarks of Repligen Corporation. POROS is a registered trademark of ThermoFisher Scientific Inc. Fractogel is a trademark of MilliporeSigma. MabSelect, SuRe, and Capto are trademarks of GE Capital. TOYOPEARL is a registered trademark of Tosoh Bioscience.
You May Also Like






