Risk-Based Biologics: CMC Flexibilities in the EU Regulatory SystemRisk-Based Biologics: CMC Flexibilities in the EU Regulatory System
March 21, 2023

Pharmaceutical companies and regulatory agencies strive to foster scientific excellence in drug development and evaluation so that members of the public can access the high-quality medicines that they need. Recent European Medicines Agency (EMA) approvals for COVID-19 vaccines highlight those organizational accomplishments and showcase the application of risk-based chemistry, manufacturing, and controls (CMC) flexibilities that are embedded within the European Union (EU) regulatory system.
Many of the CMC flexibilities outlined herein were established for PRIME (priority medicine), a scheme that the EMA launched to support and fast-track drug development. In April 2022, as the COVID-19 omicron variant spread throughout Europe, the EMA published guidance detailing a set of EU regulatory framework tools. Those tools are used for the completion of Module 3 in marketing-authorization applications (MAAs) of PRIME products that target unmet medical needs and are undergoing accelerated assessment (1).
Whether or not flexibility is exercised, products must comply with the same legal requirements for quality, safety, and efficacy. CMC requirements cannot be waived to facilitate accelerated product approval. Rather, as seen with the approval of COVID-19 vaccines, organizations can take a flexible approach. Flexibilities can be exercised toward the timing of data provision or the acceptability of using existing data from similar products in determining a positive benefit/risk judgment (2).
Supporting Priority Medicines
PRIME is an EMA scheme to enhance the regulatory support for products that address unmet medical needs. It supports drug development with a “toolbox” approach, allowing developers to use different flexibility approaches depending on their needs. The EMA’s regulatory tools and scientific elements allow for flexibility on the provision and type of CMC data, considering the overall benefit/risk and urgency of a product (1, 3). Risk-based approaches sometimes can be applied to non-PRIME products intended to address unmet clinical needs. Such approaches allow for an acceptable level of risk, depending on situational urgency (e.g., a pandemic) and a product’s clinical value. However, for products with established alternatives, the EMA is unlikely to allow CMC flexibilities.
Prior Knowledge: A Tool for Supporting CMC Flexibilities
One important support for regulatory flexibilities is the use of prior knowledge. The term prior knowledge is used in a number of guidelines (e.g., ICH Q2(R2), Q8, Q10, Q12, and Q14) established by the International Council of Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (4–8). In 2018, the EMA defined prior knowledge as “an established tool that is explicitly or implicitly used for informing decisions during pharmaceutical development and life cycle management” (9). The recently published draft ICH Q5A(R2) also includes a definition that aligns with that from the EMA (10).
The concept of prior knowledge was well established before the EMA’s formal definition, and it is used routinely during preauthorization. Prior knowledge can guide product-formulation development and justify safety-related specifications such as host-cell DNA levels. In support of CMC flexibilities, prior knowledge can help justify shifting the completion of certain studies into the postauthorization phase. It also can support alternative approaches.
Prior knowledge can come from a company’s own experience developing similar products and platforms (internal prior knowledge), or it can come from established scientific principles and technical publications outside an organization (external prior knowledge). Internal prior knowledge has been accepted by the EMA for establishing proven acceptable ranges (PARs) for manufacturing process parameters (11). For example, platform knowledge gained from the production process of one viral vector vaccine has supported the PARs of another similar vaccine.
The EMA has identified the use of external prior knowledge as a way to increase CMC flexibility for virus-reduction studies in manufacturing therapeutic monoclonal antibodies (MAbs) (9). Published prior knowledge can be used to obviate product-specific demonstration of virus reduction by multicycled protein A resin, for example. ICH Q5A(R2) shows how prior knowledge can apply to other viral-clearance steps, thereby allowing for alternative viral-clearance validation strategies. Some such flexibilities are grounded in scientific principles. For example, from a geometric standpoint it makes sense to validate the performance of small virus-retention filters with worst-case model parvoviruses instead of viruses that are several times larger. That simple step can remove days from development time.
Companies should state explicitly their use of prior knowledge when submitting regulatory dossiers to support CMC flexibility. They should justify the relevance and representativeness of that prior knowledge to the process and product in question. Companies also need to outline residual uncertainties and therefore risks that come with the application of prior knowledge, including details on how they will address those uncertainties (e.g., by deferring certain studies to the postapproval phase).
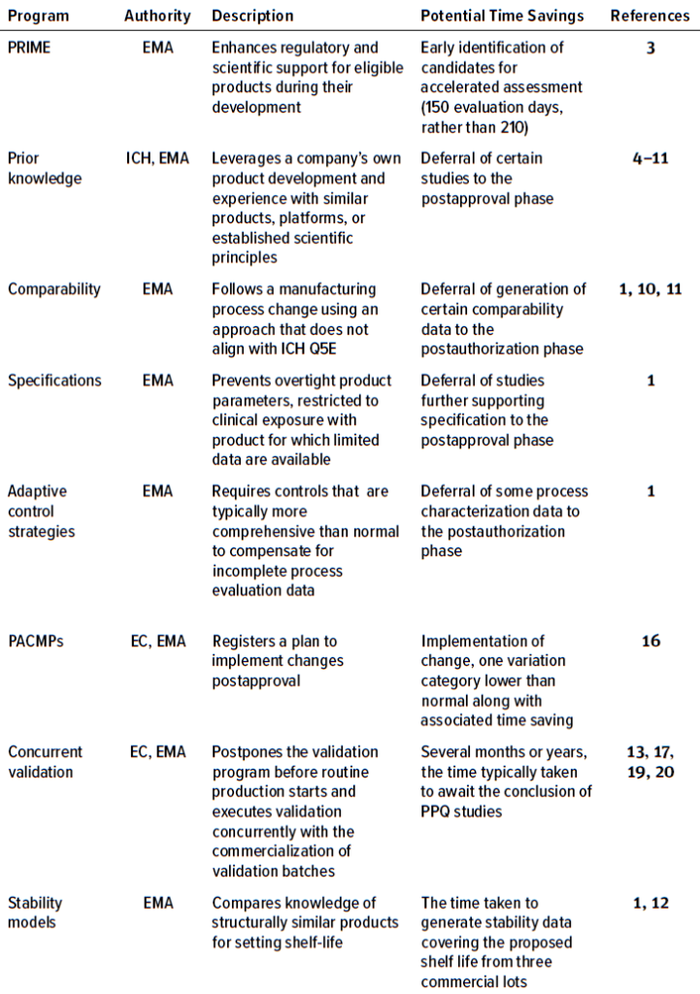
Table 1: Programs in which CMC flexibilities can be exercised in the European Union (PRIME = priority medicines, EMA = European Medicines Agency, ICH = International Council of Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, EC = European Commission, PPQ = process performance qualification, PACMP = postapproval change-management protocol).
Comparability
Comparability exercises usually are performed to support manufacturing process changes under ICH Q5E. Such exercises integrate data for both pre- and postchange material from routine batch analyses, in-process testing, production-process validation/evaluation data, characterization, and stability data. Prior knowledge often is used to predict the effect that manufacturing changes will have on quality attributes, potentially reducing the number of attributes to monitor in a comparability exercise.
That risk-based approach curates data for the exercise based on known risks to product efficacy and safety. To claim comparability following a manufacturing-process change using an approach that does not align with ICH Q5E, a company will need strong justification for doing so. Narrow or incomplete testing presents a higher risk. But in emergency circumstances such as a pandemic, details of implementing guidance for routine applications should be presented differently.
The EMA recently approved another flexible two-tiered scientific approach to demonstrating comparability of clinical and commercial material. The first tier compares a release and in-process control (IPC) results, whereas the second tier examines characterization test results (12). In brief, tier 1 data support comparability at the time of approval, and tier 2 data are requested postapproval.
Comparability data sometimes are used to support the shelf life of postchange material based on the claimed representiveness and stability data of prechange material. Recently, for development of an Ebola Zaire vaccine, the EMA requested data postapproval that demonstrated comparability between pre- and postchange materials. Such data provide evidence that a prechange clinical product is representative of the commercial product, thereby confirming its shelf life and justifying that the clinical data also apply to the commercial product (11).
Specifications
In accelerated programs or pathways for breakthrough therapies, flexibility can be exercised with some aspects of analytical procedures and acceptance criteria for release and stability testing. A flexible approach can be used to address overtight acceptance criteria that, when based on clinical exposure alone, might result in the rejection of safe and efficacious material or in assigning an unnecessarily short shelf life.
When establishing specifications for any program, not just for PRIME products, regulators will consider additional information beyond clinical experience. For example, data sources can come from in vivo studies, prior knowledge from scientific literature, or from a company’s own experience with similar products.
Acceptance criteria always should be accompanied by an appropriate clinical-impact justification. A company can base that justification on its knowledge of a product’s particular quality attributes and how those relate to safety and efficacy. Flexibility also has been exercised regarding test methods. Recently, an mRNA vaccine product was granted approval without a complete suitability demonstration of the analytical methods used to control potency and also poly-A tail length, which is important for stability of the RNA molecule and its translational efficiency. Instead, those data were requested as a postauthorization measure (13).
Adaptive Control Strategies
For biotechnology-derived products, data from successful process-evaluation studies usually are provided during the initial MAA to support the appropriateness of a proposed commercial process and its control strategy (14). That information could include production-scale clearance data to support omission of routine testing for a process-related impurity at release. Another example is the provision of process-evaluation data that demonstrate operation within a certain range to produce material that meets quality criteria.
For some products, data are too limited at the time of initial license application to justify a control strategy. In such instances, prior knowledge of a product and process can grant a company EMA flexibility in relation to a proposed control strategy, provided that the product is safe and efficacious with a positive benefit/risk balance (1). Such situations might arise for products undergoing accelerated development to address unmet medical needs, including public health emergencies. In those cases, the criticality ranking of quality attributes or process parameters might be uncertain because of limited manufacturing experience.
Such data and an associated update to the control strategy can be deferred to the postauthorization phase. In the interim, an adaptive control strategy can be applied if it is justified, offsetting the gaps in product- and process-specific knowledge. To compensate for incomplete-process evaluation data adaptive control strategies are typically more comprehensive than normal strategies Therefore, they can include additional testing at release or during processing. Adaptive control strategies also can include more process parameters than normal, with a higher number of them classified as critical and/or with tighter ranges or limits.
When additional process knowledge becomes available after a product has been approved for public use, a postapproval change-management protocol (PACMP) can be applied. Doing so registers an updated control strategy, such as the widening of ranges for process parameters. PACMPs, as outlined in the EU variations guideline (15), include detailed descriptions for proposed changes to be introduced during a product’s life cycle, along with a detailed verification explanation (16). If the proposed strategy is approved, it leads to faster postapproval implementation of the changes. Reporting changes, including the results from predefined studies, is at least one variation category lower than normal. For example, widening the limits of an approved IPC is typically a type II variation with a 60-day evaluation period because it can affect overall product quality significantly. If a change is implemented and reported in accordance with an approved PACMP, the EMA will notify the marketing authorization holder within 30 days of acknowledging receipt of the application. That approach allows for early release of a product while the details of a control strategy are finalized.
Process Validation Flexibility: Concurrent Validation
In the European Union, data from manufacturing process validation/verification studies traditionally are required before approval of a biological medicinal product. Those data often are called process performance qualification (PPQ) data. For biotechnology products based on recombinant protein expression, studies should be performed on an appropriate number (typically three) of consecutive batches produced with the commercial process and scale. Awaiting data from those studies can add several months or years to development timelines. To streamline that process, flexibility on the timing of submission data might be possible in exceptional circumstances when a strong benefit/risk balance can be justified. When that benefit/risk ratio is strong, a validation program might be postponed before the start of routine production. Then validation can proceed concurrently with commercialization of the validation batches (17). Acceptability of such an approach depends on the availability of supportive data. Therefore, data from non-PPQ and/or small-scale batches should be gathered during the developmental phase.
Concurrent validation is a strategy used when normal process validation data from replicate production runs are unavailable. That can happen when only a small number of batches have been produced (18). For example, a developer might produce one or two batches before product approval, followed by more batches postlicensure concurrent to complete the validation exercise (18). Before completion of the concurrent validation, batches can be released/used in final drug products for commercial distribution. That allowance is based on the EMA approval of a concurrent validation protocol that ensures thorough monitoring and testing of product batches. The protocol should include the IPC and process parameters that validation batches must meet before release. Such an approach might allow a company to defer submission of some validation data to the postapproval phase. The parameters and acceptance criteria included in that validation protocol control strategy must conclude that the commercial-scale process has effectively been performed and is therefore able to produce material of the intended quality.
The acceptance of concurrent validation depends on the extent of available supportive data. Thus, although supportive data from non-PPQ batches produced using a commercial process might demonstrate control of the manufacturing process, approval of a concurrent approach requires justification. Developers should justify that the process evaluation studies performed represent the commercial process. In addition, supportive data can be generated from batches made to supply clinical studies. Therefore, during development, biomanufacturers should focus on gathering sufficient data on those supportive developmental lots. Justification should accompany such data, addressing the representiveness of the material for the commercial process. The use of prior knowledge from similar products or platforms can be used as supportive data, if applicable, such as when companies determine which validation parameters and associated acceptance criteria to include in the concurrent protocol.
In August 2021, the EMA granted a conditional marketing authorization for a therapeutic monoclonal antibody (MAb) used to treat a rare disease. The use of a concurrent approach for the validation of chromatography-column resin and tangential-flow filter lifetimes is detailed in an assessment report, running concurrently with the product’s commercial manufacturing (19). The concurrent approach has been approved for several other products, including a COVID-19 pandemic vaccine (13) and a gene therapy for a rare disease (20).
Stability Models
Stability testing of biotechnological/biological products typically is performed in line with relevant ICH guidance (Q1/Q5C), which involves collecting stability data that cover the proposed shelf life for biological medicinal products from three different lots. Collected data should be representative of the manufacturing and storing processes. Collection can be time consuming for product developers, especially when development and approval timelines are compressed. Therefore, in cases where data have yet to be generated and their collection would lengthen a timeline substantially, companies sometimes can set a product shelf life by applying prior stability knowledge of structurally similar products (1). That “stability modeling” approach has been used during the recent approval of some vaccines (12).
In cases with few available time points from stability studies, a company can base its product’s shelf life on platform data from similar products if doing so can be adequately justified. That is a statistical modeling approach, wherein the following should be considered and provided for review: drug substance stability, shelf-life specifications, expected losses during filling, temperature excursions during product use, and an estimate of degradation slopes. Unless strongly justified otherwise, the structurally similar product on which the model is based should be stored under the same conditions, in the same type of container, possessing the same stability-indicating quality attributes, and should be evaluated using the same analytical methods. Approval of such an approach is likely to require the provision of real-time stability data generated after approval. Doing so would ensure that a product’s stability continues to fit the model-based prediction. As required for all products, companies must report all deviations from the expected specification results.
Enabling Patient Accessibility
When applied correctly, regulatory flexibilities for Module 3 can significantly accelerate development of medicines addressing high unmet needs. These are important tools to help patients gain timely access to life-saving treatments and have specifically proven their value during the COVID-19 pandemic. It is important that such tools are applied correctly, because omission of mandatory CMC could lead to delays. Knowledge of regulatory expectations thorough risk analysis and evaluation of the benefit/risk balance are key to applying CMC flexibilities successfully.
References
1 EMA/CHMP/BWP/QWP/IWG/
694114/2019. Toolbox Guidance on Scientific Elements and Regulatory Tools To Support Quality Data Packages for PRIME and Certain Marketing Authorisation Applications Targeting an Unmet Medical Need. European Medicines Agency: Amsterdam, The Netherlands, 22 April 2022; https://www.ema.europa.eu/en/documents/scientific-guideline/toolbox-guidance-scientific-elements-regulatory-tools-support-quality-data-packages-prime-certain_en.pdf.
2 Shivji R, et al. Considerations for the Chemistry, Manufacturing and Controls (CMC): Quality Package for COVID-19 Vaccines — Interim Lessons Learnt by the European Medicines Agency. Vaccine 40(38) 2022: 5539–5541; https://doi.org/10.1016/jvaccine.2022.06.058.
3 PRIME: Priority Medicines. European Medicines Agency: Amsterdam, The Netherlands, 3 February 2023; https://www.ema.europa.eu/en/human-regulatory/research-development/prime-priority-medicines.
4 ICH Q2(R2) (Draft). Validation of Analytical Procedures. US Fed. Reg. 87(166) 2022: 52784–52786; https://database.ich.org/sites/default/files/ICH_Q2-R2_Document_Step2_Guideline_2022_0324.pdf.
5 ICH Q8(R2). Pharmaceutical Development. US Fed. Reg. 75(152) 2010: 48199; https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf.
6 ICH Q10. Pharmaceutical Quality System. US Fed. Reg. 74(66) 2009: 15990–15991; https://database.ich.org/sites/default/files/Q10%20Guideline.pdf.
7 ICH Q12. Technical and Regulatory Considerations for Pharmaceutical Product Lifecycle Management. US Fed. Reg. 86(96) 2021: 27437–27438; https://database.ich.org/sites/default/files/Q12_Guideline_Step4_2019_1119.pdf.
8 ICH Q14. Analytical Procedure Development. US Fed. Reg. 87(106) 2022: 52784–52786; https://database.ich.org/sites/default/files/ICH_Q14_Document_Step2_Guideline_2022_0324.pdf.
9 EMA/CHMP/BWP/187162/2018. Meeting Report: Joint BWP/QWP Workshop with Stakeholders in Relation to Prior Knowledge and Its Use in Regulatory Applications. European Medicines Agency: Amsterdam, The Netherlands, 23 November 2017; https://www.ema.europa.eu/en/documents/report/meeting-report-joint-biologics-working-party/quality-working-party-workshop-stakeholders-relation-prior-knowledge-its-use-regulatory-applications_en.pdf.
10 ICH Q5A(R2) (Draft). Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. US Fed. Reg. 87(218) 2022; https://database.ich.org/sites/default/files/ICH_Q5A%28R2%29_Step2_draft_Guideline_2022_0826.pdf.
11 EMA/606159/2019. Ervebo Assessment Report. European Medicines Agency: Amsterdam, The Netherlands, 17 October 2019; https://www.ema.europa.eu/en/documents/assessment-report/ervebo-epar-public-assessment-report_en.pdf.
12 EMA/158424/2021. COVID-19 Vaccine Janssen Assessment Report. European Medicines Agency: Amsterdam, The Netherlands, 11 March 2021; https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-janssen-epar-public-assessment-report_en.pdf.
13 EMA/707383/2020. Comirnaty Assessment Report (Corr.1*1). European Medicines Agency: Amsterdam, The Netherlands, 19 February 2021; https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf.
14 EMA/CHMP/BWP/187338/2014. Guideline on Process Validation for the Manufacture of Biotechnology-Derived Active Substances and Data To Be Provided in the Regulatory Submission. European Medicines Agency: Amsterdam, The Netherlands, 28 April 2016; https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-process-validation-manufacture-biotechnology-derived-active-substances-data-be-provided_en.pdf.
15 2013/C 223/01. Guidelines on the Details of the Various Categories of Variations, on the Operation of the Procedures Laid Down in Chapters II, IIa, III and IV of Commission Regulation (EC) No 1234/2008 of 24 November 2008 Concerning the Examination of Variations to the Terms of Marketing Authorisations for Medicinal Products for Human Use and Veterinary Medicinal Products and on the Documentation to be Submitted Pursuant to Those Procedures. Off. J. of the Euro. Union: Mercier, Luxembourg, 8 February 2013; https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52013XC0802%2804%29.
16 EMA/CHMP/CVMP/QWP/586330/2010. Questions and Answers on Post Approval Change Management Protocols. European Medicines Agency: Amsterdam, The Netherlands, 30 March 2012; https://www.ema.europa.eu/en/documents/scientific-guideline/questions-answers-post-approval-change-management-protocols_en.pdf.
17 Annex 15: Qualification and Validation. EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. European Commission: Brussels, Belgium, 30 March 2015; https://health.ec.europa.eu/system/files/2016-11/2015-10_annex15_0.pdf.
18 ICH Q7. Good Manufacturing Practice for Active Pharmaceutical Ingredients: Scientific Guideline. European Medicines Agency: Amsterdam, The Netherlands, 10 November 2000; https://www.ema.europa.eu/en/ich-q7-good-manufacturing-practice-active-pharmaceutical-ingredients-scientific-guideline#current-effective-version-section.
19 EMA/426468/2021. Minjuvi Assessment Report. European Medicines Agency: Amsterdam, The Netherlands, 24 June 2021; https://www.ema.europa.eu/en/documents/assessment-report/minjuvi-epar-public-assessment-report_en.pdf.
20 EMA/CHMP/571076/2022. Upstaza Assessment Report. European Medicines Agency: Amsterdam, The Netherlands, 19 May 2022; https://www.ema.europa.eu/en/documents/assessment-report/upstaza-epar-public-assessment-report_en.pdf.
David W. Murray, PhD, is a subject matter expert (SME) of biological medicinal products and regulatory affairs. He is a principal consultant at Parexel International Ireland Ltd., Kilmainham, Dublin, Ireland; [email protected]; https://www.parexel.com.
You May Also Like






