A SciLog® MabTec™ Method for Manual Bolus Fed-Batch Versus MabTec™ Automated Continuous Fed-BatchA SciLog® MabTec™ Method for Manual Bolus Fed-Batch Versus MabTec™ Automated Continuous Fed-Batch

SciLog’s MabTec™ is an add-on bioreactor maintenance system that can gravimetrically manage, automate, and document your bioreactor feeding or perfusion strategy. The MabTec™ upgrades many manual processes to walk-away automation with minimal investment in terms of capital or time.
Summary
MabTec™ combines accuracy with convenience to provide an ideal solution for cell culture feeding strategies. Based on the results, MabTec™ demonstrated the ability to increase protein production while eliminating several hours of manual daily operation.
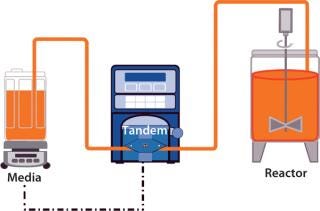
Figure 1: ()
Introduction
The application flexibility of MabTec™ delivers superior growth efficiency within a bioreactor through the replacement of repetitive manual operator steps with an automated solution. The replacement allows for more constructive use of operator time and eliminates the inadvertent errors associated with manual production. The objective of the experiment is to
Demonstrate the feasibility of replacing a manual fed batch process with an automated process.
Verify automated performance results against manual operations. Automated results must meet or exceed manual operations.
Determine the number of manual operations that could be eliminated with an automated process.
Test Scenario
Three ten liter (10 L) glass bioreactors were filled with four liters (4 L) of media to be used in a mammalian cell culture. Agitation was started and maintained at the same rate for the duration of the run. After five (5) days the culture reached a density point where a feeding strategy is required.
The manual feeding method required an operator to perform ten percent (10%) bolus reactor fluid additions daily. Each day’s bolus media quantity required the operator to autoclave the media feed container and prep the media daily. The automated feeding method was setup to add media to the reactor in a continuous method that totaled a ten percent (10%) daily reactor weight addition. All the media for the automated process was prepared at one time and placed on a cart next to the MabTec™. The run was scheduled for a total of eighteen (18) days. The automated method was allowed to continue for an additional three (3) days as this method of processing had not been tested previously. The reactor was sampled and tested daily for viable cell density, percentage cell viablity, protein concentration, glucose, lactate, glutamine, and ammonium.
Results
Initial conditions were extremely similar for all three reactors. % Viabilities for all three reactors on day five (5) was ninety-eight percent (98%) or better when the switch to automated fed batch began.
The three (3) runs were not significantly different, which was the intended outcome for the test. The automated MabTec method was able to reproduce the manual method exactly and added consistency to the method that was not possible before.
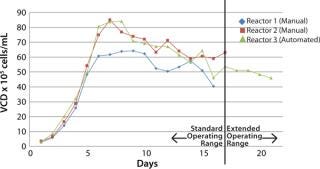
Figure 2: ()
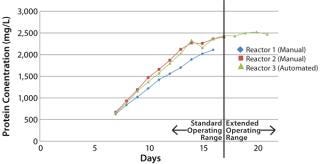
Figure 3: ()
Figure 4: ()
Table 1: Data from three runs, each after day 5

Table 1: Data from three runs, each after day 5 ()
Conclusion
The viable cell densities produced in the bioreactor fed by the MabTec™ was on par with the two (2) manual methods. The MabTec™ also allowed slightly higher protein production than the other two (2) vessels. The switch to an automated process eliminated thirty (30) manual operations, fifteen autoclave cycles, and fifteen (15) buffer preparations, which equated to several hours of operator time freed up. The MabTec™ has demonstrated its feasibility to replace a manual fed-batch operation.
<
div style=”font-size:71.429%;”>About the Author
Author Details
Dean Pighin is engineering manager, and Donald Beers is sales engineer at SciLog, 801 Deming Way, Madison, WI 53717; 1-800-955-1995; [email protected]; www.scilog.com/MabTec. Copyright © Scilog 2012. All Rights Reserved
You May Also Like






