Continuous Biomanufacturing: A New Approach to Process ScaleContinuous Biomanufacturing: A New Approach to Process Scale

(WWW.PALLBIOTECH.COM)
The BioPhorum first-edition Technology Roadmap outlined a 10-year vision for therapeutic protein production in the biopharmaceutical industry (1). The roadmap describes multiple manufacturing scenarios ranging from large-scale (~20,000-L production) to small and agile, portable production facilities. It includes detailed analyses of the needs for the future in each of the following areas:
Process technologies (2)
Inline monitoring and real-time release (3)
Automated facilities (4)
Modular and mobile (5)
Knowledge management (6)
Supply partnership management (7).
Since the 2017 publication of the technology roadmap, the BioPhorum Technology Roadmapping Phorum has prioritized key needs outlined in it and put into place a series of follow-on activities designed to accelerate the technology development that the roadmap describes. At the same time, further detail is being added to the roadmap documents, and a vision of the future continues to be developed. The next analysis on continuous bioprocessing will be published soon, with particular emphasis on downstream manufacturing.
Continuous bioprocessing has been proposed as one future biomanufacturing state because it operates at similar scales for both clinical and commercial production using flexible facilities that can react readily to changing market pressures. Application of flexible infrastructure such as singleuse flow paths and small surge vessels and similar bioreactor sizes will reduce footprint and capital investment while simplifying technology transfer activities and maintaining quality-attribute profiles. Continuous equipment trains can operate over long periods, so this manufacturing approach will need closed processing to manage the risk of microbial contamination. In addition, integration of downstream processes into a single flow path requires synchronization of each unit operation through automated process control.
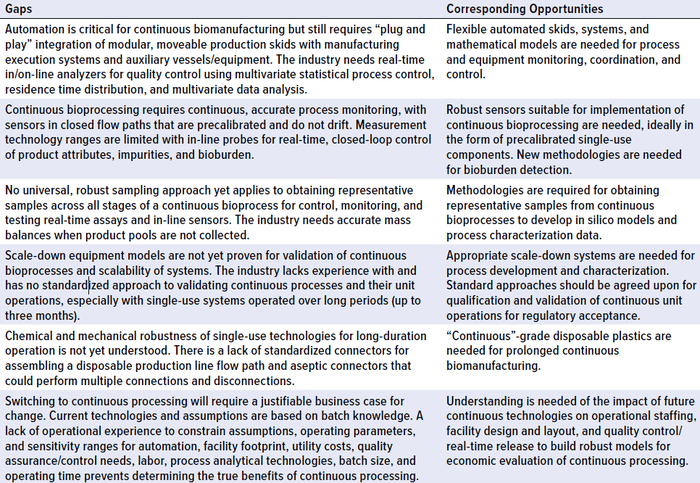
Table 1: Summary of identified hindering gaps and opportunities for adoption of continuous biomanufacturing
BioPhorum has identified technology and regulatory gaps for continuous downstream drug-substance processing using a monoclonal antibody (MAb) as a model system. Because the manufacturing processes for many different biotherapeutics use identical or similar purification technologies, process monitoring and control, and regulatory frameworks, findings in the analysis paper are applicable to other protein therapies. Many biomanufacturers are investing in research programs and laboratory-scale demonstrations of continuous bioprocessing, but few have transitioned to clinical or manufacturing scale. The overview paper identifies a number of gaps hindering the implementation of continuous downstream drug substance biomanufacturing for clinical or commercial production of therapeutic proteins to encourage the industry to find solutions to those challenges (Table 1).
Analysis performed by the BioPhorum team complements and builds on the first-edition technology roadmap — in particular by further describing technologies outlined in the process technologies chapter, with respect to a continuous manufacturing environment. The group drew from a combination of technology, process, business modeling, and regulatory expertise from across the BioPhorum roadmapping community to ensure that the analysis would cover a broad range of recommendations. Although the process technologies chapter outlined future needs with respect to each unit operation of a bioprocess, the new document deals more holistically with challenges associated
with a continuous bioprocess overall. The intention is to complete further analysis by prioritizing needs described to identify opportunities in which development may be accelerated by collaboration.
The process modeling described in the first-edition technology roadmap has been reviewed so that the needs of a continuous process can be highlighted. Opportunity remains for further development of the model to help us understand where continuous processing may be of the most value. The first-edition process model described manufacturing scenarios of the future, seeking to highlight areas of opportunity where technology acceleration can break through process bottlenecks. The following excerpt from the first-edition roadmap overview (8) describes that modeling work and conclusions drawn and identifies opportunities for future analysis.
References
1 Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/phorum/technologyroadmapping.
2 Process Technologies. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/process-technologies.
3 In-Line Monitoring and Real-Time Release. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/in-line-monitoring-and-realtime-release.
4 Automated Facility. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/automated-facility.
5 Modular and Mobile. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/modular-and-mobile.
6 Knowledge Management. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/knowledge-management.
7 Supply Partnership Management. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www. biophorum.com/supply-partnership-management.
8 Overview. Technology Roadmapping. BioPhorum: London, UK, 2017; https://www.biophorum.com/overview.
Mark Brower is a principal scientist at Merck MSD, Charlie Heise is a subject matter expert in primary separations and filtration at FUJIFILM Diosynth Biotechnologies, Graeme Moody and Clare Simpson are BioPhorum facilitators.
You May Also Like






