- Filtration
- Sponsored Content
- Vaccines
Filter-Based Clarification of Viral Vaccines and VectorsFilter-Based Clarification of Viral Vaccines and Vectors
Sponsored by Millipore Sigma
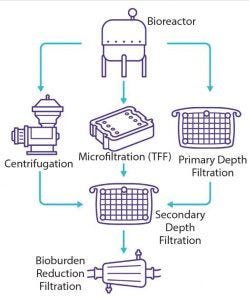
Figure 1: Usual clarification options for viral vaccines and vectors
Viral vaccines rely on the antigen properties of a virus or virus-like entity to trigger an immune response and induce immune protection against a forthcoming viral infection. Through development of recombinant viral vaccines, developers can reduce risks associated with the presence of live and inactivated viruses. Instead, recombinant vaccines induce immunity against a pathogen by relying on the capacity of one or more antigens delivered by means of viral vectors or the baculovirus/plasmid system (1). Viral vaccines are formulated with or without adjuvants and categorized as shown in Table 1.

Table 1: Classification of viral vaccines
Viral vaccine manufacturing processes can be templated. They follow a general scheme, starting with production in either an embryonated egg or mammalian/insect cell culture. After production, the bulk harvest material is processed to purify a vaccine of interest. Following upstream production and lysis (optional), a clarification step typically is introduced to start the purification process by either centrifugation or filtration. This is a critical unit operation because it strongly affects product recovery and subsequent downstream purification.
Selection of the clarification methodology depends on the type of cells involved, the nature of the virus, and properties of the process fluids. Filtration-based technologies have gained prominence in vaccine clarification following the increasing implementation of single-use technologies upstream. Filtration methods include membrane technology (microfiltration operated in normal-flow filtration (NFF) mode, tangential-flow filtration (TFF), or depth filters operated as NFF.
Here we provide a comprehensive overview of different filtration technologies and their application in viral vaccine clarification. We also outline challenges and present current best practices.
Considerations for Clarification of Viral Vaccines: Type of Substrate
Composition, type, and level of contaminants to be removed during clarification mainly depend on the upstream process and expression system used for production. In most cases, the virus particles must be kept integral during the clarification step.
For decades, embryonated chicken eggs have been used to produce both human and animal vaccines. However, the resulting allantoic fluid harvest (rich in virus particles and cellular debris) is a challenging feed for clarification. This fluid has a high solids content (>25%, which increases with embryo age) and high mineral and protein content, hence its highly viscous consistency. It also contains rudimentary tissue compounds from chicken embryos such as feathers, beaks, blood vessels, and/or blood cells.
Several viral vaccines have moved away from an egg-based process toward the use of live cells (e.g., plant, microbial, avian, or mammalian). The composition of these feed streams can vary significantly. Based on cell viability — and lysis method selected (e.g., chemical or mechanical), if applicable — the impurity profile in the fluid to be clarified can differ considerably. For example, low cell viability can indicate high levels of contaminants released into a feed stream from cell lysis. Typical contaminants such as host cell DNA, host cell proteins (HCPs), lipids, and bigger particles such as cell debris can be identified. The proportion of solids in the feed usually is an indicator of purifying challenges ahead (~6–8% mammalian cells or up to 40% in yeast). Compared with allantoic fluid, however, cell culture harvests are considerably cleaner in terms of solids load and soluble content.
Another expression method is the baculovirus expression vector system (BEVS) used with insect cells. This is gaining interest particularly for producing viral vectors and virus-like particles (VLPs). Other expression systems such as bacteria, yeast, and plant cells also can be used to produce viral particles.
Considerations on Key Product Quality Criteria and Control Strategies
Yield: In most cases, yield is an off-line measurement conducted at the end of a number of process steps. Depending on the success criteria for each step, yield can be the main parameter to consider when selecting one option over another for a given step. Based on the size and properties of viral particles, yield could be affected by the clarification method used. For example, whereas positive charges increase nucleic acids and HCP capture, diatomaceous earth can retain viruses by adsorption.
Some viruses are shear sensitive and can be damaged by high shear exposure in disk-stack centrifuges or by high cross-flow and multiple pump passages through TFF. Due to their large size, viruses larger than 100 nm also can be retained simply by tight filters. Thus, companies should take such factors into consideration when selecting depth filters because some depth filter devices include a 0.1-μm membrane that could cause retention-driven product loss. Other process elements such as aggregation and an excess of impurities can compromise viral particle recovery.
Final Product Purity Levels: Regulatory agencies provide recommendations and requirements regarding acceptable residual amounts of contaminants in final drug products. For reasons of patient safety and tolerance, host cell DNA in a final product must be reduced to appropriate levels. In 1998, the World Health Organization (WHO) specified the maximum residual DNA content in a vaccine to be <10 ng/dose. Since then, the European Medicines Agency (EMA) proposed more stringent conditions based on the type of cell line (tumorigenic origin) used in vaccine manufacturing. The US Food and Drug Administration (FDA) follows a case-by-case evaluation approach and recommends that manufacturers reduce both the size (~200 bp) and amount of DNA per dose.
To date, a final DNA content of <10 ng/ dose commonly is accepted for most biologics. Similarly, a recombinant monoclonal antibody (MAb) product must reach clearance of impurities to <100 ppm of HCP, ≤10 ng/dose of DNA, and <5% of immunogenic aggregates (Table 2).
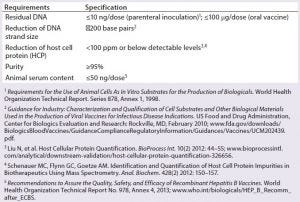
Table 2: Acceptable remaining impurities in vaccine products
Feed Quality Evaluation Criteria and Processing Parameters: Several parameters can be used to assess the clarity or quality of a product, either during process development or after each manufacturing process step. Turbidity is an easy parameter to monitor and provides an immediate assessment of feed quality. For example, it enables the detection of depth filter breakthrough.
The two primary methods of ascertaining the effectiveness of the clarification step are centrifugation and filtration. Turbidity can be monitored simply by absorbance/scatter in the visible range, providing an immediate assessment of particle load within the filtrate or centrate. Turbidity also relates to filter capacity, which is the volume of feed a depth filter can process before the pressure drop breaches specifications. Capacity relating to both pressure drop and turbidity breakthrough are linked, and specifications for both should be set during process development. For some processes (particularly those with a smaller average particle size in the feed), turbidity breakthrough is the limiting factor in sizing a filtration train. Often, capacity limit is attributed to pressure drop. But because high pressures or flow rates can cause premature turbidity breakthrough, both mechanisms can be related.
Technology Options for Clarification of Virus Vaccines: Because of the extreme diversity of viral vaccines in terms of size, structure, shape, and expression system, no unified template exists for their production and purification. Those processes can be divided into four different phases: upstream/production, clarification, purification, and formulation.
To reduce burden on downstream purification steps, the main objective of a clarification process is to remove undesirable materials, including whole cells, cell debris, colloids, and large aggregates. As the first downstream process, clarification should be optimized to maximize product yield and purity. Several serial operational steps often are required to achieve a desired level of clarification. The first operation (often referred to as primary clarification) removes larger particles, and the second (often referred to as secondary clarification) removes colloids and other submicron particles (Figure 1).
In theory, all available technologies (low-speed centrifugation, microfiltration TFF, NFF) can be selected and potentially combined to clarify viruses. As with other manufacturing process steps, a clarification process should have predictable scalability, be manufacturing-friendly (e.g., be easy to use, reduce holdup volume, provide operator safety), and have a low cost of goods (CoG). However, the clarification process of viral vaccines has two unique characteristics that can require more tailored solutions:
low solids content with a high nucleic acid and colloid content, requiring higher retention capacity
high feed variability and cell culture enhancements, requiring more robustness (2).
Although centrifugation can handle a high solids load and traditionally has been used in batch and continuous modes, it requires large capital investment and high maintenance costs. More important, centrifugation scale-up can be problematic because of unreliable scale-down models with nonlinear scalability and high-shear operation for shear-sensitive vaccines. But NFF and TFF have gained interest for vaccine clarification because they are significantly easier to scale-up and implement.
NFF: Primary clarification using NFF typically involves depth filters that often contain positively charged material and filter aids (e.g., diatomaceous earth) that improve retention of cell debris, colloids, and negatively charged contaminants. NFF relies on two main mechanisms for particle retention: size exclusion and adsorption.
NFF membrane filters can be used in secondary filtration because they retain particles by size exclusion. Certain grades of depth filters have a tighter pore-size distribution (which offers greater colloidal particle retention) but do not have high holding capacity. Noncharged depth filters also can be used for clarification while offering higher cost-effectiveness for small batches (≤1,000 L). They typically use three media types:
melt-blown media fabricated in a pleated format to achieve higher flow rates and holding capacities
graded density of concentrically wrapped media to allow the filter to remove finer contaminants progressively
membranes to provide higher retention efficiency.
TFF membranes with retention ratings in the range of 0.1–0.65 μm have been used to retain cells, cell debris, and other large contaminants. Most TFF devices are linearly scalable and reusable after cleaning, and hence greatly reduce consumable costs. However, certain viruses (e.g., extracellularly produced enveloped virus-like particles) can be damaged by high-shear exposure in disk-stack centrifuges or by high cross-flow and multiple pump passages in TFF processes. Open-channel TFF devices (cassette format without screen) are preferred to minimize shear.
Case Studies
Egg-Based Vaccines: In influenza vaccine processes, a typical allantoic fluid harvest is rich in proteins (e.g., ovalbumin, lysozyme, ovomucin) and contains 45 μg hemagglutinin antigen per egg (~3–4 μg HA and 108–109 infectious units/mL of allantoic fluid). The typical gravity-settled turbidity of a virus-containing allantoic fluid (VCAF) generally is 46–132 NTU. Low-speed zonal continuous centrifugation around 4,000–5,000g often is the preferred option to remove large particles and thus gets used for primary clarification, typically providing a recovery yield of 70% (3, 4). Many vaccine manufacturers use sucrose gradient zonal centrifugation to purify and concentrate viruses. However, polypropylene and cellulose-based depth filters also can be implemented for filtration. Fair capacities of 150–210 L/m² and up to 3× reduction of feed stream turbidity can be achieved with those allantoic fluid harvests. That option is appropriate for influenza vaccines, which are prone to adsorption loss during clarification on charged filters.
NFF also can be used for secondary clarification. Combinations of polypropylene, cellulose, and glass-fiber materials generally demonstrate good efficiency (5). Using a salt solution can reduce association between a virus vaccine and solid debris, resulting in a yield increase of about twofold without compromising viral particle integrity.
One study demonstrated that higher ionic strength on allantoic debris increased influenza virus yields (6). In that case, 1.5 M NaCl was applied to pooled allantoic fluids of various influenza strains, and the sample was centrifuged for different durations to understand the amount of virus partitioned in the supernatant and pellet. Control samples were kept at 0.15M NaCl. This test scope was expanded to include other influenza strains — A/New Caledonia (H1N1), A/Texas (H3N2), B/Jiangsu and B/Hong Kong — to demonstrate an average twofold yield increase. Further work verified the integrity of the purified virus in control and higher ionic strengths. Data showed that higher ionic strength did not adversely affect the live titer of the tested influenza virus strains (2). Specifically, studies have reported use of a 1.2-μm cellulose nitrate (CN) filter polypropylene media followed by 0.45-μm filter polyvinylidene fluoride (PVDF) membrane for clarification of cell-based influenza with a loading of 111 L/m² and 105 L/m², respectively (7).
Another option is use of TFF with a 0.65-μm or 0.45-μm microfiltration membrane device operated with permeate flux control (8). Using a “two-pump process” with a permeate pump in addition to the standard TFF feed pump allows for permeate flux control to manage/reduce polarization and fouling. That provides better characterization of the “critical” flux — the limiting flux above which a process becomes unstable (9).
Moreover, researchers conducted primary clarification of allantoic fluid using a 40-μm bag filter followed by an open-channel microfiltration device (Prostak 0.65 μm; data not shown). Using a crossflow of 3 LPM/channel, with transmembrane pressure (TMP) at 0.2 bar and dP at 0.4 bar, a capacity of 33 L/m² was achieved. The MF process was designed for 10× concentration and 5× diafiltration, and the Prostak allowed for 60× reuse.
Viruses in Adherent Cells on Microcarriers: Microcarriers can be used as a support matrix for the growth of adherent cells such as Vero cells. Primary clarification of these cells grown on microcarriers can be performed using a 75-μm stainless steel sieve to remove microcarriers from harvest. A Millistak+ C0HC depth filter medium then can be used for secondary clarification and directly followed by a sterilizing-grade filter (10). This study was reported for a cell density of 0.78 x 106 cells/mL. Results showed varying filter capacity based on cell density or cell viability of harvest.
Viral Vectors: Trial results on lentiviruses from the supernatant of HEK293T cells show positive performance (data not shown). The negative charge of lentiviruses is known to be responsible for poor recoveries with positively charged depth filters. Therefore, despite the fact that no sign of plugging was observed using a Millistak+ C0HC depth filter, very low viral vector recovery was recorded. However, use of a 1.0/0.5 μm Polysep II filter led to both a high capacity and high 84% recovery. The Polygard CN filter, on the other hand, behaved quite well in terms of recovery (75%) but showed more signs of plugging. An extra 10–20% lower virus recovery should be considered for the following 0.45 μm or 0.22μm (sterile) filtration step.
Two other studies on lentivirus feed streams confirm the positive recovery performance using the Polygard CN filters (CN25, CN12, CN10, and CN06 with more than 80% recovery). Other studies report an interesting result with recoveries reaching up to 90% for the Millistak+ CE50 filter that does not contain inorganic filter aid (e.g., diatomaceous earth; data not shown).
Adenoviruses also can be prone to adsorption, but divergent results have been reported. In some cases, good adenovirus recovery is observed even when a positively charged depth filter medium containing diatomaceous earth such as the Millistak+ HC medium is used (11). If adenovirus is lost, Polygard and Clarigard filters can be used instead, but a secondary clarification might be needed to reduce turbidity (12).
Clarification Options
Different approaches are used to produce viral vaccines, making designing a typical template for their clarification difficult. Indeed, these products can be produced by different expression systems and possess a range of physicochemical properties. The clarification method selected should take those factors into account to ensure that yield and contaminants removal are sufficient.
NFF and microfiltration TFF technologies are increasingly preferred to centrifugation because of their more predictable scalability and robustness. Low CoG also can be achieved by tailoring the choice of media chemistry and porosity to gain high recovery and productivity with satisfactory impurity removal. Continuing innovation in this field by suppliers of purification and filtration systems will help vaccine manufacturers meet their current and future challenges on the road to developing novel and efficient therapies.
References
1 Nascimento IP, Leite LCC. Recombinant Vaccines and the Development of New Vaccine Strategies. Braz. J. Med. Biol. Res. 45(12) 2012: 1102–1111.
2 Besnard L, et al. Clarification of Vaccines: An Overview of Filter-Based Technology Trends and Best Practices. Biotechnol. Advances 34(1) 2016: 1–13.
3 Hendriks J, et al. An International Technology Platform for Influenza Vaccines. Vaccine 29 Suppl 1, 2011: A8–11.
4 Eichhorn U. Influenza Vaccine Composition. US Patent US7316813 B2, 2008.
5 Lampson GP, Machlowitz RA. Process for Producing Purified Concentrated Influenza Virus. US Patents US3547779 A, 1970.
6 Hughes K, et al. Yield Increases in Intact Influenza Vaccine Virus from Chicken Allantoic Fluid through isolation from Insoluble Allantoic Debris. Vaccine 25(22) 2007: 4456–4463.
7 Thompson M, Wee J, Nagpal A. Methods for Purification of Viruses. European Patent EP2334328 A4, 2012.
8 Lau SY, et al. Impact of Process Loading on Optimization and Scale-Up of TFF Microfiltration. BioProcess J. 13(2) 2014: 46–55; doi:10.12665/J132.Pattna.
9 Raghunath B, et al. Best Practices for Optimization and Scale-Up of Microfiltration TFF Processes. Bioprocess. J. 11(1) 2012: 30–40.
10 Thomassen YE, et al. Scale-Down of the Inactivated Polio Vaccine Production Process. Biotechnol. Bioeng. 110(5) 2013: 1354–1365.
11 Namatovu HH, et al. Evaluation of Filtration Products in the Production of Adenovirus Candidates Used in Vaccine Production: Overview and Case Study. BioProcess J. 5(3) 2006: 67–74.
12 Weggeman M, van Corven EJJM. Virus Purification Methods. US Patent: US8124106 B2, 2012.
Corresponding author Anissa Boumlic ([email protected]) is associate director of the vaccine segment at Millipore S.A.S.
You May Also Like





