Growth Projections for the Regenerative Medicine MarketGrowth Projections for the Regenerative Medicine Market
February 13, 2017

Already have an account?
Figure 1: World regenerative medicine market by product type, 2015–2022 ($million) (1)
One of the fastest growing medical markets with great potential, regenerative medicine treats chronic diseases that were once untreatable. Therapy using live cells is increasingly used to replace damaged tissue, deliver gene therapies to target tissues and organs, and stimulate self-healing along with a number of other applications.
View the full article below – Login Required
Stem cell therapies, a segment of cell-based therapies, hold huge market potential to revolutionize the treatment modality for regenerating or repairing diseased organs. Advancement in regenerative technologies has fueled the commercial applications of stem cells in the world regenerative medicine market for both allogeneic and autologous therapies.
Gene therapy involves transplanting genes into a patient’s cells to replace those genes that are leading (or could lead) to particular diseases such as certain cancers or genetic disorders. Such treatments make up a small portion of the regenerative medicine market, but the gene therapy segment is expected to grow (Figure 1) as research methods into personalized medicines improve.
Similar to stem cell therapies, biomaterials and tissue engineering also are gaining popularity. Bioprinting research is showing steady progress, with increasing demand for engineered tissues and organs (2). Three-dimensional (3D) bioprinting is the process of culturing cells or other biocompatible materials to build tissues and organs. Biofabrication strategies produce materials that mimic the features of the original tissue and organs and can potentially replace them, thus curing the diseased or damaged parts of a patient’s body.
Progress and Limitations
The limited availability of donated tissue and organs for transplantation in patients with acute conditions has augmented market growth. Moreover, increasing availability (and decreasing cost) of 3D technologies and nanotechnologies could address the unmet needs of organ transplantation. However, the breakthrough of novel technologies to achieve whole human organs is still elusive. That is where bioprinting technology is currently making progress with advancements in cell therapies, tissue engineering, and the growing field of 3D bioprinting.
Extensive R&D activities now are being carried out for regenerative medicine using novel technologies. Such products could revolutionize the healthcare industry, with an ultimate goal of producing complex internal organs such as livers, kidneys, and hearts. For instance, Organovo Holdings, Inc. (San Diego, CA) is experimenting to produce liver tissue for preclinical toxicity testing and drug screening using its NovoGen 3D bioprinting process. The company launched exVive3D human liver tissue for preclinical drug discovery testing in 2014. That helps companies test their drug product candidates for potential liver toxicity and check adsorption, distribution, metabolism, and excretion (ADME) outcomes.
Emerging new technologies in regenerative medicine are set to be adopted universally, thanks to the rising incidence of health issues (especially organ failures) and an increasing gap between the number of donors and patients requiring organ transplants.
However, several issues are limiting market growth: high costs of the most technologically advanced products, unanticipated state of improving technologies, long production times, and viability of bioengineered cells. One major disadvantage of regenerating a complete new organ using adult stem cells is rejection of the replaced tissues in the human body. In addition, exhaustive research is required for construction of complicated tissues and organs that can mimic original organs. Nevertheless, growing R&D investments in regenerative medicine is creating new opportunities for key players to regenerate vital human organs such as livers, kidneys, and hearts.
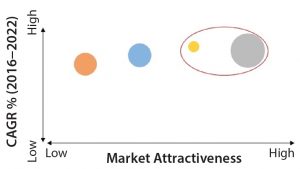
Figure 2: Market attractiveness for investors, forecast for 2016–2022; CAGR = compound annual growth rate
Marketplace and Growth
Organovo is a pioneer in the field focused on 3D bioprinting of human tissues using multiple cells such as fibroblasts, muscle cells, endothelial cells, and others. Spectrum Health (Grand Rapids, MI, with a heart model), Maipu Regenerative Medical Technology (Guangdong, China, with dura mater products for brain surgeries), and others are persistently conducting research and development (R&D) in this field. Such extensive R&D should help the industry overcome its current challenges and discover novel technologies for regenerative medicine, with the potential for customizable and even off-the-shelf offerings of whole-transplant organs in the coming years.
Major players in the world regenerative medicine market (Figure 1) have adopted acquisition and product approval as their key developmental strategies to meet increasing demands for regenerative medicine in areas such as dermatology, neurodegenerative diseases, orthopedics, and cardiovascular diseases. Companies are implementing novel concepts and ideas with increasingly effectual manufacturing techniques and improving their available product portfolios. That helps enhance their profitability to gain a competitive edge on the global market. Some companies have received approvals from the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), the Australian Therapeutic Goods Administration (TGA), and other regulatory agencies for launching new, technologically advanced products and expanding their product offerings across different geographical areas.
Other key companies have adopted different strategies for market growth. For instance, Athersys, Inc. (Cleveland, OH) and Healios KK (Tokyo, Japan) partnered to develop and commercialize regenerative cell and tissue therapies. Integra Life Sciences Holdings Corporation (Plainsboro, NJ) received US FDA approval for Integra dermal regeneration template for the treatment of diabetic foot ulcers. This approval enhanced the wound care product portfolio of the company. Cryolife (Kennesaw, GA) acquired privately held medical device company On-X Life Technologies Holdings, Inc. That brought it access to mechanical heart-valve technology and provided entry to the valve market for tissue engineering.
Reference
1 Regenerative Medicines Market: Global Opportunity Analysis and Industry Forecast, 2015–2022. Allied Market Research: Pune,Maharashtra, India: August 2016; www.alliedmarketresearch.com/regenerative-medicines-market.
2 Chartrain NA, Williams CB, Whittington AR. Engineering Tissues with Bioprinting. BioProcess Int. 14(10) 2016: 28–33.
Swapna Supekar is senior digital marketing executive at Allied Market Research in Pune, Maharashtra, India; [email protected].
About the Author
You May Also Like






