Supply and Demand Trends: Mammalian Biomanufacturing Industry OverviewSupply and Demand Trends: Mammalian Biomanufacturing Industry Overview
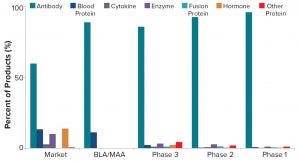
Figure 1: Distribution of mammalian-cell–based products by product type and phase of development (BLA = biologics license application, MAA = marketing authorization application)
Since the 1986 approval of the first recombinant therapeutic antibody, OKT3, biopharmaceuticals have become a large percentage of overall pharmaceutical company revenue. In 2018, sales of the top five selling recombinant proteins — Humira (adalimumab, AbbVie), Keytruda (pembrolizumab, Merck), Herceptin (trastuzumab, Genentech), Enbrel (etanercept, Amgen), and Avastin (bevacizumab, Genentech), all antibodies — totaled over US$48 billion. The compound annual growth rate (CAGR) for antibodies revenue was about 20% from 2004 to 2014. Those products include naked monoclonal antibodies (MAbs), Fc-fusion proteins, antibody fragments, bispecific antibodies, antibody conjugates, and other antibody-related products. However, that CAGR has slowed to the midteens because of the maturation of many biopharmaceutical products and emerging alternative therapeutic modalities. And such growth rates are difficult to sustain as overall market size increases.
Projections of Product Demand
To provide context for this growing segment of the biopharmaceutical market, the Bioprocess Technology Group’s (BPTG’s) proprietary bioTRAK database of products and biomanufacturing capacity estimates that nearly 1,400 biopharmaceutical candidates are in some stage of clinical development in the United States or Europe. Nearly 85% of those are produced in mammalian cell cultures. We evaluated the distribution of products based on mammalian-cell culture by type and phase of development to refine the biomanufacturing market further. Figure 1 shows product distribution by phase of development for antibodies, blood proteins, cytokines, enzymes, fusion proteins, hormones, and other recombinant proteins. Nearly 60% of commercially marketed products are antibodies, and they are the largest product type for all phases of development, with the early stage pipeline consisting of nearly all antibody products. It is important to note that many early commercial biopharmaceutical products such as growth hormones, insulins, and interferons are produced in microbial systems.
Whether commercially approved or still in development, all product developers need access to mammalian-culture production capacity. For current commercially approved biopharmaceuticals, future demand is estimated from each product’s reported annual sales data, along with estimates of future growth rates. Our future product growth estimations take product age into consideration, because sales growth typically slows as a product matures. Newly approved products often do not reach full market penetration for several years.
Projected treatment population size is estimated based on sales and price per milligram. Combining the size of the population with the yearly per-patient dosing, we forecast the kilogram quantities required to meet demand of each product for the next five years. Those forecasts can be converted to liter quantities for each product using cell-line expression amount and overall purification yield estimates.
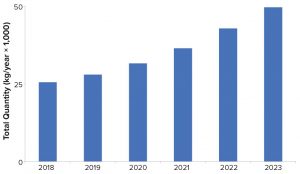
Figure 2: Estimated bulk kilograms of product needed to meet product demand
Our estimates are based on industry benchmarks at the time that products were being developed and the process maturity of those products’ companies. For example, the commercial process for a product launched over 10 years ago is likely to have a lower expression level assigned in our forecast algorithm than a product that is currently in clinical development. For products in development, future commercial demand is estimated based on market penetration of currently approved products or proxy products with similar indications. For products in development, we used a phase-based commercialization probability assumption when calculating future demand.
Figure 2 shows the projected kilogram quantities of product needed to meet annual commercial and clinical demand for all drug types made using mammalian-cell cultures. In 2018, about 25 metric tons of product were required. As more biopharamceuticals enter the pipeline and receive commercial approval, the overall kilogram requirements needed to meet demand are likely to increase from about 25 metric tons in 2018 to nearly 50 metric tons in 2023.
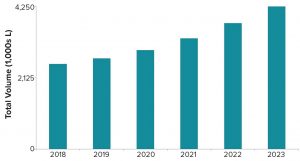
Figure 3: Estimated volumetric capacity needed to meet product demand
Figure 3 shows the projected volumetric capacity needed to meet annual commercial and clinical demand for all product types produced using mammalian-cell systems. In 2018, annual volumetric requirements were just over 2,500 kL. That value is projected to increase to over 4,200 kL by 2023, representing a five-year growth rate of 11%.
As with any forecasting model, our assumptions are based on the most-probable scenarios and include estimations for biopharmaceuticals in development for certain large patient population indications such as Alzheimer’s disease or broad cancer treatments such as PDL/PDL-1 checkpoint inhibitors. Should several of those large-demand products obtain regulatory approval and adequate reimbursement by healthcare oversight organizations (e.g., US pharmacy benefit managers or the UK National Institute for Healthcare and Excellence (NICE)) or become part of a managed-entry agreement between a company and public payer of a social or national health insurance system, then demand for manufacturing capacity could increase significantly, potentially leading to a serious capacity shortage.
Conversely, other manufacturing trends could decrease demand for some biomanufacturing capacity. Among these are the industry’s increased focus on orphan indications, a shift from full-length naked antibodies to alternative formats, and more potent products (e.g., antibody–drug conjugates (ADCs) and bispecific antibodies), which would require lower doses. Given the projected increase in volumetric demand over the next five years, the industry is cognizant of the inherent volatility of production capacity forecasts. A degree of uncertainty exists in balancing demand and supply because of production problems, market demand fluctuations over time, regulatory and reimbursement issues, and competitive factors.
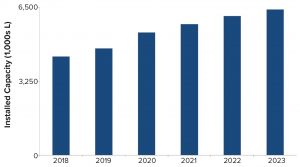
Figure 4: Manufacturing capacity for products based on mammalian-cell culture
Capacity Forecasts
To understand how well the biomanufacturing industry is positioned to meet future product demands, we estimated the 2018 mammalian-cell–culture supply to be nearly 4,400 kL and predicted it to grow to about 6,400 kL by 2023, a five-year growth rate of 8% (Figure 4). However, not all capacity is equally available throughout the industry. In 2018, product-development companies controled over 70% of the installed mammalian-cell–culture capacity. By contrast, hybrid companies (those developing products, but also selling or making available excess manufacturing capacity) and contract manufacturing organizations (CMOs) controlled significantly less capacity. Distribution of capacity is likely to change slightly by 2023, with product companies controlling 65% of the installed capacity, CMO capacity increasing by 6%, and hybrid companies remaining stable with a 1% increase.
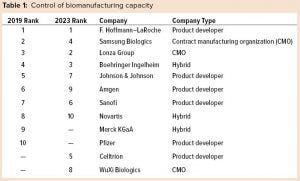
Although product development companies control most cell-culture capacity, the distribution of this capacity is highly concentrated within 10 companies (Table 1). Capacity for companies not ranked in the top 10 is distributed among nearly 130 companies in 2019, and probably will reach nearly 135 companies in 2023. Currently, nearly 65% of the capacity is controlled by 10 companies; in 2023, this value will change to <60%. Based on substantial capacity investments, Celltrion and WuXi Biologics are likely to displace Merck KGaA and Pfizer from the top 10.
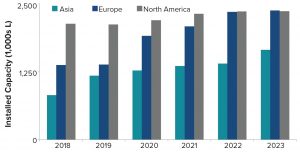
Figure 5: Geographic distribution of biomanufacturing capacity
Figure 5 shows the geographic distribution of manufacturing facilities. In 2018, nearly half of all mammalian-cell–culture capacity was in North America, followed by Europe and Asia. Over the past five years, however, capacity in North America and Europe has grown modestly whereas capacity in Asia has increased significantly. By 2023, with strong growth rates projected in Asia (~9%) and Europe (~15%), North America and Europe will have equivalent capacity. Capacity growth particularly in Korea and Singapore as well as Ireland is probably the result of government incentives and tax advantages, among other factors.
 As described earlier, different drug products require different capacities. For example, the 2018 kilogram demand for the top-five–selling antibody products totaled nearly 6.8 metric tons. The demand for the more than 90 remaining marketed antibody products combined was about 15 metric tons. For products still in development in a best-case commercial scenario in which market success and maximum market penetration are assumed, projected demand for nearly 60% is expected to be <100 kg per product per year. Only 10% of the products — such as those for Alzheimer’s disease, Parkinson’s disease, diabetes, and possibly coronary heart disease and atherosclerosis — are projected to require >750 kg/year.
As described earlier, different drug products require different capacities. For example, the 2018 kilogram demand for the top-five–selling antibody products totaled nearly 6.8 metric tons. The demand for the more than 90 remaining marketed antibody products combined was about 15 metric tons. For products still in development in a best-case commercial scenario in which market success and maximum market penetration are assumed, projected demand for nearly 60% is expected to be <100 kg per product per year. Only 10% of the products — such as those for Alzheimer’s disease, Parkinson’s disease, diabetes, and possibly coronary heart disease and atherosclerosis — are projected to require >750 kg/year.
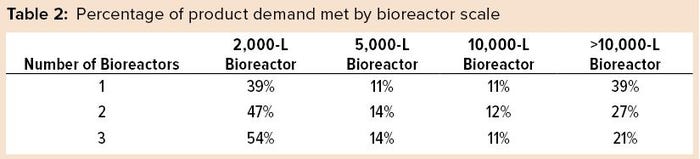
A closer review of future projected commercial manufacturing demands for products in phase 2 and 3 clinical development reveals that half of them are likely to be met with a single 2,000‑L or 5,000-L bioreactor, assuming 18 batches per year per bioreactor and a 90% success rate for batch manufacturing (Table 2). However, large-scale capacity still will be needed. Our model predicts that the remaining half of products will need bioreactor capacity of 10,000-L and greater to meet forecasted demand. Increasing the number of bioreactors increases the manufacturing capacity and unsurprisingly causes a shift in the percentage of products for which development are likely to be met. For example, a single 2,000-L bioreactor can manufacture 39% of drug products in phase 2 and phase 3 whereas three bioreactors at that scale can manufacture over half (54%) of drug in development.
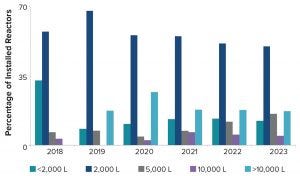
Figure 6: Percentage and scale of future bioreactors
If we analyze the cumulative number and scale of bioreactors coming on line between 2018 and 2023 at the <2,000-L, 2,000-L, 5,000-L, 10,000-L, and >10,000-L scales (Figure 6), it is evident that most bioreactors projected to come on line are 2,000 L. Nearly 20% of bioreactors are at a scale of 10,000 L or greater. Biomanufacturers understand capacity-demand scenarios and are installing equipment to meet anticipated needs.
Strong Growth in Future
Overall, the biopharmaceutical industry will continue to have strong growth for the foreseeable future, and antibody products will be the dominant driver of that growth. Currently, installed capacity can meet biomanufacturing demand for these products, but control and location of capacity can affect accessibility. Most capacity is product-developer rather than CMO based, which could make it difficult for companies without their own capacity to access it at the right time and under the right terms. North America has the greatest percentage of installed capacity, but Asia and Europe have seen a surge in new capacity installations.
 Although capacity will increase over the next five years, demand for capacity will increase at a slightly faster rate, allowing for some short-term loosening of capacity constraints. After 2023, those will be tightened. In recent years, we have noted that the industry experienced some capacity constraints at clinical scales because of high clinical demand, and the industry responded with a wave of facility expansions. The type and scale of equipment being installed also will be important because the demand for half of the products in mid- to late-stage development can be met with 5,000 L of capacity or less. The remaining half will need larger capacity to meet future demands. With new bioreactor installations reflecting future demand profiles, we are focused on watching how the industry is responding to such demands for capacity to ensure that current and future biopharmaceutical products will be available to patients.
Although capacity will increase over the next five years, demand for capacity will increase at a slightly faster rate, allowing for some short-term loosening of capacity constraints. After 2023, those will be tightened. In recent years, we have noted that the industry experienced some capacity constraints at clinical scales because of high clinical demand, and the industry responded with a wave of facility expansions. The type and scale of equipment being installed also will be important because the demand for half of the products in mid- to late-stage development can be met with 5,000 L of capacity or less. The remaining half will need larger capacity to meet future demands. With new bioreactor installations reflecting future demand profiles, we are focused on watching how the industry is responding to such demands for capacity to ensure that current and future biopharmaceutical products will be available to patients.
Corresponding author Dawn M. Ecker is director of bioTRAK database services at BioProcess Technology Group, BDO-USA, LLP; [email protected]. Patricia Seymour is managing director at BioProcess Technology Group, BDO -USA, LLP, One International Place, Boston, MA 02110; [email protected]; https://www.bdo.com/industries/life-sciences/bioprocess-technology.
Having first appeared in the CPhI annual report from Informa (BPI’s parent company), this article is reprinted here with permission and edited for BPI style. Download the full report at https://www.cphi.com/europe/visit/news-and-updates/annual-industry-report-2019-final.
You May Also Like






