BioXcellence BI-HEX® Mammalian Expression PlatformBioXcellence BI-HEX® Mammalian Expression Platform

Figure 1: ()
The production of therapeutic proteins like monoclonal antibodies (MAbs) and bispecifics depends on mammalian expression due to the posttranslational modifications occurring in these molecules. At Boehringer-Ingelheims (BIs) BioXcellence unit, we use the BI-HEX™ mammalian expression platform for fast and cost-efficient development of high-titered manufacturing processes. The BI-HEX™ platform is composed of the following interlinked elements: BI’s proprietary vector system, the BI-HEX™ cell lines, which are derived from the CHO-DG44 cell line (1), a short and efficient manufacturing process, as well as BI proprietary media and feeds. Use of automation and robotic systems as well as miniaturization of many of the processes used during cell line, upstream, and downstream development are key features of the platform. For example, the ambr system was recently introduced for downscaled fed-batch clone screening.
The BI-HEX™ platform is versatile in the sense that it is made of variations of each element and therefore allows for selection of the right components for a given product. For example, a panel of BI-HEX™ expression vectors is at hand made of different genetic elements and frameworks. Likewise, the platform includes two different substrains of the BI-HEX™ host cell line, HEX1 and HEX2. Both cell lines are derived from the CHO DG44 cell line, but they differ in their glycoprofiles. HEX1 has a higher content of the A2FG0 and also a higher level of defucosylated carbohydrates compared with HEX2, which has a higher A2FG1 content (Figure 1).
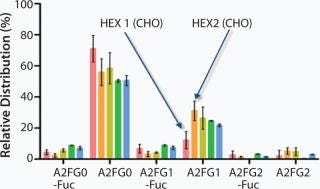
Figure 1: ()
The glycoprofile of a MAb is known to influence the effector function of the molecule, not least with respect to antibody-dependent cell cytotoxicity (ADCC). ADCC is enhanced in MAbs with a low fucose content in the N-linked carbohydrate structure attached to Asn297 in the constant region of the molecule. Depending on the desired function of a therapeutic antibody, we select either HEX1 or HEX2 as our host cell line starting point. For development of biosimilars or in case of a cell line change during clinical development, where it is critically important to match the originator molecule product quality attributes, the availability of different host cell sublines provides the opportunity to preselect the one where the product quality profile is foreseen to be most similar to the originator molecule.
Starting from our platform manufacturing process and media composition, we use a fast and robust design of experiments (DoE) approach for further optimization. We typically see significant increases in titer during this part of the optimization and titers of 5–8 g/Lhave been reached with BI-HEX with animal-component–free, chemically defined media.
Variation of the media composition, timing of the feed, and hardware process paramenter will all have an impact not only on titer, but also on product quality. Thus, it allows us to influence different critical product quality attributes, such as the glycan composition or the CD16 binding activity, which makes it an essential tool not least for biosimilar development.
A very exciting addition to the BI-HEX™ platform is the engineered BI-HEX GlymaxX cell line that results in production of antibodies with very low levels of fucose. This is achieved by means of the GlymaxX™ technology developed by ProBioGen with whom BI has entered into a collaboration agreement in 2011. The BI-HEX GlymaxX antibodies exhibit a very significant increase in ADCC activity. This is an attractive feature for example for therapeutic antibodies intended for treatment of cancer.
About the Author
Author Details
Corresponding author Dr. Anne Tolstrup is director of cell culture II, Barbara Enenkel is associate director molecular biology; Stefan Schlatter, Jochen Schaub, and Harald Bradl are associate directors of cell culture development; Anja Puklowski and Patrick Schulz are scientists; Anurag Khetan is director cell culture I; and Hitto Kaufmann is vice president process science Germany, +49-7351- 54-141353; fax +49-7351-54-92159; [email protected].
REFERENCES
1.) Urlaub, G, and LA. Chasin. 1980. Isolation of Chinese Hamster Cell Mutants Deficient in Dihydrofolate Reductase Activity. Proc. Natl. Acad. Sci. 77:4216-4220.
You May Also Like





