Plant-Based Protein Expression: Emerging Systems Bring Viable Approaches to Biopharmaceutical ManufacturingPlant-Based Protein Expression: Emerging Systems Bring Viable Approaches to Biopharmaceutical Manufacturing

GravityFlow automated multilayer vertical farm systems at an Intravision Greens facility in Niagara, Canada. HTTPS://WWW.INTRAVISIONGROUP.COM
The application of plant-based systems to produce biopharmaceuticals for human and veterinary indications is a rapidly expanding field. Available systems range from stable transgenic root-cell culture to transient expression in whole plants. Products that have been expressed include monoclonal antibodies (MAbs) (1), subunit vaccines (2), virus-like particles (VLPs) (3), specialty enzymes (4), and structural proteins such as collagen (5). “Traditional” bioproduction platforms such as Chinese hamster ovary (CHO) cells, Escherichia coli, and Pichia pastoris have long histories of patient safety and regulatory acceptance. Novel systems based on cells from insects (transfected with baculovirus), transgenic animals, and plants are quickly accumulating the clinical safety data required to provide similar confidence for use (6).
The State of Plant-Based Protein Expression
One of the first approved products derived from plant-based processes was a vaccine against Newcastle disease in chickens (7). That product was derived from plant cell culture and was approved by the US Department of Agriculture (USDA) in 2006. Currently, one product has received full approval from the US Food and Drug Administration (FDA) for human use: Protalix BioTherapeutics’s Elelys (taliglucerase alpha), a human glucocerebrosidase produced in carrot-root cell culture (8). Mapp Biopharmaceutical’s ZMapp MAb cocktail for Ebola treatment was produced in Nicotiana benthamiana (9). The drug was approved for compassionate use during the 2014 outbreak in Western Africa. A subsequent clinical trial demonstrated its safety and therapeutic benefit but failed to reach the specified statistical threshold for efficacy, largely due to the low number of enrolled patients (10).
Results of a phase 3 trial were recently reported for a VLP influenza vaccine produced in N. benthamiana by Medicago Inc. The study demonstrated the product’s safety and efficacy as well as the consistency of its production process (11). Early in 2022, Medicago’s Covifenz vaccine against SARS-CoV-2, based on plant-derived VLPs, received approval from Health Canada for use in adults 18–64 years old (3, 12).
The Case for Plant-Based Systems: The specific advantages claimed for plant-based biopharmaceutical production include low production costs, speedy development, scalability, and inherent safety. Growth of plants requires only air, light, water, and fertilizer salts (though plant cell culture in bioreactors requires addition of a suitable carbon source such as glucose to support growth). Thus, plant-based systems eliminate the need for costly supplements that are required for mammalian cell culture. Using whole plants as hosts enables significant reductions in facility costs compared with bioreactor-based manufacturing systems. Plant-based production systems also provide potential benefits for scalability: Increasing manufacturing scale requires only that more plants be grown, rather than necessitating installation of ever-larger bioreactors and associated infrastructure (steam, water, and sanitary pipework).
Plant-based production systems greatly reduce risks for inadvertent transfer of infectious agents (such as latent viruses, prions, or transmissible nucleic-acid molecules) from host to patient, as compared with platforms based on mammalian cells or transgenic animals. In addition, using plant systems can significantly shorten development timelines for new products. For example, using a transient expression platform, the time between learning a MAb’s protein sequence and having gram-scale quantities of substantially pure product suitable for testing in a small-animal model can be as brief as four weeks (13). Thus, plant-based systems also enable developers to change the product being manufactured relatively quickly. Such platforms have short inoculum-development chains compared with the typical multistage bioreactor train required for mammalian expression systems.
Available Hosts and Approaches: Several recent reviews summarize the status of plant-based expression for production of biopharmaceuticals (14–16). Many systems are available depending on plant type (rice (17); tobacco and potato (18); and lettuce (19))and localization of the protein product (cytoplasm, apoplast, chloroplast, or vacuole (20)). Which option to select depends upon the characteristics required for the final product. For example, MAbs typically are secreted into the extracellular space (apoplast) via the endoplasmic reticulum (ER)/Golgi secretory system, then recovered from plant extracts using standard filtration and chromatographic approaches. Claimed yields have reached up to four grams of product per kilogram of plant biomass (15). In other cases, product proteins can be retained within plant tissue — e.g., encapsulated in chloroplasts. Minimal processing of the plant biomass yields a feed additive that can be formulated for oral delivery (19).
There are two basic approaches to heterologous protein expression in plant cells: transient and stable transgenic expression. Transient systems often use the common soil bacterium Agrobacterium tumefaciens for gene delivery, whereby rapid expression can be achieved over a relatively short time before natural plant responses reduce expression from foreign DNA sequences. Transgenic systems rely upon the integration of recombinant DNA into the host genome for long-term expression of a product. Transgenic plants are created using A. tumefaciens infection or DNA-coated particle bombardment and subsequent regeneration of plantlets from infected cells (calli). These plantlets are bred to homozygosity for active DNA inserts (which, by definition, are relatively benign in terms of plant physiology and development). This is similar to the process for creation of production cell lines for mammalian expression systems — and likewise can be time consuming.
In the case of parenteral drug products, or when there is need for a rapid response, transient expression systems using N. benthamiana generally are preferred because they generate high yields and use an industry-standard host (21). To produce encapsulated drugs destined for oral delivery, a stable transgenic platform might be preferable. That would use food-grade plants in which the product gene is included in the plant (or plastid) genome.
A transient system depends upon the ability of A. tumefaciens to transfer genes into a plant host through a tumor-inducing (Ti) plasmid vector (22). There are many variants of this system, depending on what specific elements are included in the vector along with the gene(s) of interest. In general, the choice can be reduced to use of viral components for in situ replication and systemic distribution of the genes (23) or use of nonviral methods (24). Herein, we focus on transient expression platforms using N. benthamiana as a host and with a nonviral system based on a binary transfer DNA (T-DNA) vector.
In summary, the gene for the protein of interest is cloned into a T-DNA vector and transformed into a suitable strain of A. tumefaciens (e.g., EHA105 and LBA4404). In a process known as agroinfiltration, the transformed bacteria are introduced to plant tissue to transfer the product gene into the plant cells (Figure 1). After incubation, plants are harvested and homogenized. Proteins are extracted by physical (filtration) and chemical (affinity chromatography) methods to provide a drug substance with the required purity. In practice, each component of the overall process has multiple requirements and presents opportunities for optimization, as outlined in the following sections.
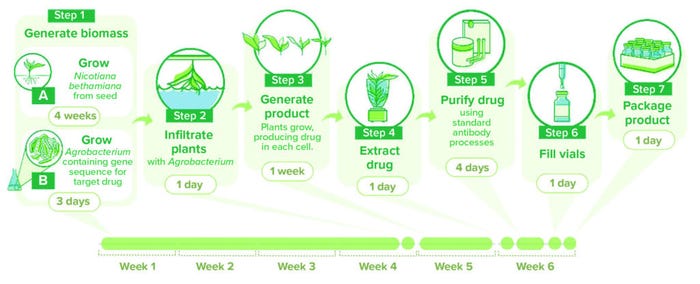
Figure 1: Schematic of a plant-based production process using transient expression
Plant-Based Protein Expression At a Glance
Gene Design and Vector Construction: Depending on the source of the original DNA sequence for a protein of interest, it might be advantageous to modify codon use to mimic that of the host plant. Several online tools apply codon bias for N. benthamiana to create a synthetic DNA sequence for any protein. The novel sequence can be scanned to eliminate potential intron splice sites, micro RNA (miRNA) motifs, and polyadenylation sequences — among other problematic sequences that are context specific. However, a gene’s intron structure can be designed specifically to provide for intron-mediated enhancement of expression (25). In the case of proteins that will be secreted from plant cells, native or plant-preferred variations in signal peptide can be used, with the option of targeting proteins to specific compartments such as vacuoles (26). Designed genes are cloned into the T-DNA region of a suitable binary vector in the context of control elements including a promoter, 5′ and 3′ untranslated regions (UTRs), and a terminator, each of which will dramatically affect final product yield (27, 28).
Once the sequence is verified, a vector construct is transformed into
A. tumefaciens using standard chemical methods and/or electroporation. Stocks of the strains to be used for manufacturing are maintained according to a formal cell banking system that complies with regulatory guidelines.
Growth of A. tumefaciens: The Agrobacterium strain(s) to be used for expression of a protein of interest typically are grown in a standard bacterial culture medium to a stage at which they are stable and infective (29). Industrial processes normally use well-controlled bioreactors, although laboratory-scale developmental studies routinely use shake flask systems (30). In processes destined for production of human therapeutics, culture media should be formulated to contain no animal-derived components (31). Plant-derived compounds, such as phenolics produced by wounded tissue or their analogs (e.g., acetyl syringone), frequently are used in growth media to enhance bacterial infectivity, but they are not essential (21). Conditions for growth such as pH, temperature, and dissolved oxygen as well as time of harvest will influence product yield from the plant tissue (32).
Growth of N. benthamiana: Plant growth conditions before exposure to Agrobacterium strains are critical to a production process. Factors such as growth substrate (nutrient content, pH, and salinity), incident light, photoperiod, leaf temperature, atmospheric CO2 concentration, humidity, and air-flow patterns will all affect plant health and morphology — which, in turn, will affect a plant’s interaction with infective Agrobacterium and consequently influence protein yield (33).
Early stage development work frequently is conducted in greenhouse systems with limited environmental control. They can be subject to uncontrolled variability, which affects yield and quality of recombinant proteins (produced either transiently or from transgenic plants) (34). In an industrial setting, and especially for biopharmaceuticals produced under current good manufacturing practice (CGMP) conditions, plants typically are grown in highly controlled contained systems based on multilayer hydroponic stacks (as pictured on the first page of this article). Lighting is provided by controlled low-temperature light-emitting diodes (LEDs) to enable zoned definition of parameters of intensity and spectral quality depending on plant age and needs. Such facilities can achieve high productivity in a small footprint and are constructed for cleanability and controlled access. The use of robotic systems to move trays of plants onto and out of the stacks provides further opportunity for control and efficiency.
A particular advantage of transient expression is that commitment to manufacturing a specified product is made at the preinfiltration stage of plant growth. The plants, in effect, are product agnostic until they are exposed to the A. tumefaciens carrying the product vector(s). That provides for exceptional flexibility in a manufacturing facility.
Agroinfiltration: The process for placing Agrobacterium in contact with host plant cells first involves dilution of high-density bacterial cultures in a suitable buffer. Although plants can withstand surprisingly high concentrations of Agrobacterium infiltration cocktails, the optimal number of viable, infective cells required in a cocktail to maximize the number of gene copies transferred to each cell should be identified by experimentation. In the case of multigene systems, the individual components can be delivered through single strains containing many genes (35); multiple strains, each containing a single vector (36); or some combination of those. In effect, the decision is both practical (“What works best?”) and economic (“What costs the least?”). For example, multigene vectors might exhibit fragility during gene transfer from bacterium to plant cell, and use of several individual gene strains will require multiple bioreactors, which will impose significant process costs.
The infiltration process itself requires that a system efficiently introduce bacterial strains to the interior of a leaf or to a whole plant (via stomata). Whole plants in a tray are transferred from a growth chamber to a pressure vessel containing the bacterial suspension, typically using conveyor systems to limit human intervention. The plants are inverted and immersed in the suspension, and chamber atmospheric pressure is reduced to expel air from the leaves. After a short period, pressure is returned to normal, and the leaves fill with bacterial suspension to begin the process of DNA transfer into the plant cells through a bacterium’s type 4 secretion systems (37). Infiltrated plants are removed from the pressure chamber and transferred to a second set of growth stacks for postinfiltration incubation. Any systems involved in the mechanical handling of plants before and immediately after infiltration must be designed to limit physical damage to those plants.
Postinfiltration Incubation for Product Expression: As for initial plant growth, plants that have been exposed to Agrobacterium and infected with foreign DNA are allowed to incubate under appropriate conditions in a contained environment while they accumulate product. The incubation conditions (including time) that support highest yields of recoverable product of appropriate quality might be different from those that were best for preincubation growth, so such conditions should be determined empirically. For example, environmental temperature or light intensity might need to be reduced to limit accumulation of nonproductive (noninfected) biomass. However, care must be taken to prevent stress responses, which can significantly reduce yield or quality of a heterologous protein. A CGMP-compliant facility will feature a linear process flow with increasing environmental containment from preinfiltration plant growth through agroinfiltration to plant harvest and transfer to downstream product recovery.
Product Recovery: There is little practical difference between product recovery from plant-based systems and that from other, well-established product expression platforms. Product-containing biomass is homogenized in an appropriate buffer (with protease inhibitors and/or antioxidants, depending on the requirements, to maintain product integrity through processing) and filtered to remove insoluble components and debris. The process stream may be ultrafiltered and/or concentrated before the first chromatography step, all under conditions that are designed to prevent contamination with adventitious agents (bacteria and fungi) (38).
Special Considerations
The plant-based protein production systems outlined above also require some particular considerations. One involves plant responses to the pathogen insult inherent to transient Agrobacterium-mediated infection. A plant will mount a response to limit damage by the foreign DNA and/or the presence of bacteria in the leaf tissue. That response can take several forms, including production of inhibitors of bacterial integrity such as salicylic acid or phenolic compounds.
The most significant response of concern is that against the foreign DNA inside infected plant cells. This is similar in effect to the antiviral response termed posttranslational gene silencing (PTGS), whereby molecular mechanisms are assembled to detect and destroy the mRNA driving heterologous protein production (39). Plant cells use small RNA molecules created from the mRNA (through activities of systems such as Dicer endoribonuclease) and incorporated into the RNA-induced silencing complex (RISC) to direct degradation of the parent molecule. Further systems act on the DNA to depress activity at the promoter regions by methylation (40).
A solution to that problem lies in the means used by viruses to counter the plant defense. Several viral proteins are known to interfere with PTGS responses. For example, the P19 protein of tomato bushy stunt virus is among the most efficient, widely used, and studied suppressors of PTGS (41).
Host plants might also respond to foreign proteins by producing proteases, which can reduce protein yield significantly and/or affect product quality (42). Protease responses can be addressed either by using inhibitors or by deleting specific genes from the plant genome. N. benthamiana has a unique characteristic: It sacrifices viral defense for rapid growth — which makes the species particularly useful for biotechnology applications (43).
Plants will glycosylate secreted proteins, mainly at N-linked sites and in much the same way as found in mammalian expression systems. O-glycosylation, however, is not commonly reported (44). Glycans added to secreted proteins in plants are different from those of CHO cells. Plant glycans have a biantennary structure similar to that of glycans found on animal cells. However, plant glycosylation pathways do not add terminal galactose or sialic acid efficiently. Both of those can be added by coexpressing relevant glycosyltransferases along with the protein of interest. Moreover, plants do not add the human-type α-1,6-fucose at the protein-proximal N-acetyl glucosamine residue. Instead, they typically add an α-1,3-fucose at that position. There is a second plant-specific addition of β-1,2-xylose to the branch-point mannose.
Those plant-specific glycan features are potentially immunogenic (45) and must be removed from biotherapeutic protein products that are destined for treatment of humans. Removal can be achieved by down-regulating (46) or deleting the cognate fucosyl- and xylosyltransferases (e.g., using techniques based on clustered regularly interspaced short palindromic repeats (CRISPR) and associated protein 9 (Cas9)) (47).
Viable Approaches
Plant-based expression of proteins and specialty chemicals is a rapidly expanding application of industrial biotechnology. Such platforms have been used extensively for accelerated production of diagnostic reagents (48), vaccines (49), and therapeutics (50) in response to the current SARS-CoV-2 pandemic. The understanding of the underlying biology required to maximize efficiency and breadth of application of the plant-based production platform is increasing exponentially, but much work remains to be done.
References
1 McLean M. Trastuzumab Made in Plants Using vivoXPRESS Platform Technology. J. Drug Des. Res. 4(5) 2017: 1052–1055; https://www.jscimedcentral.com/DrugDesign/drugdesign-4-1052.pdf.
2 LeBlanc Z, Waterhouse P, Bally J. Plant-Based Vaccines: The Way Ahead? Viruses 13(1) 2021: 5; https://doi.org/10.3390/v13010005.
3 Gobiel P, et al. Phase 2 Randomized Trial of an AS03 Adjuvanted Plant-Based Virus-Like Particle Vaccine for COVID-19 in Healthy Adults, Older Adults and Older Adults with Comorbidities. medRxiv 14 October 2021; https://doi.org/10.1101/2021.05.14.21257248.
4 Uthailak N, et al. Transient Production of Human β-Glucocerebrosiade with Mannosidic-Type N-Glycan Structure in Glycoengineered Nicotiana benthamiana Plants. Front. Plant Sci. 12, 2021: 683762; https://doi.org/10.3389/fpls.2021.683762.
5 Shoseyov O, et al. Human Collagen Produced in Plants. Bioengineered 5(1) 2014: 49–52; https://dx.doi.org/10.4161%2Fbioe.26002.
6 Shanmugaraj B, Bulaon CJI, Phoolcharoen W. Plant Molecular Pharming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 9(7) 2020: 842; https://doi.org/10.3390/plants9070842.
7 The First Plant-Derived Vaccine Approved for Chickens. Pharm. Executive 14 February 2006; https://www.pharmexec.com/view/first-plant-derived-vaccine-approved-chickens.
8 Fox JL. First Plant-Made Biologic Approved. Nature Biotechnol. 30(6) 2012: 472; https://doi.org/10.1038/nbt0612-472.
9 Qiu X, et al. Reversion of Advanced Ebola Virus Disease in Nonhuman Primates with ZMapp. Nature 514(7520) 2014: 47–53; https://www.nature.com/articles/nature13777.
10 PREVAIL II Writing Group. A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N. Engl. J. Med. 375(15) 2016: 1448–1456; https://doi.org/10.1056/NEJMoa1604330.
11 Ward BJ, et al. Phase III: Randomized Observer-Blind Trial to Evaluate Lot-to-Lot Consistency of a New Plant-Derived Quadrivalent Virus Like Particle Influenza Vaccine in Adults 18–49 Years of Age. Vaccine 39(10) 2021: 1528–1533; https://doi.org/10.1016/j.vaccine.2021.01.004.
12 Medicago Covifenz COVID-19 Vaccine. Health Canada: Ottawa, Ontario, 31 March 2022; https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html.
13 Powell JD. From Pandemic Preparedness to Biofuel Production: Tobacco Finds Its Biotechnology Niche in North America. Agriculture 4(5) 2015: 901–917; https://doi.org/10.3390/agriculture5040901.
14 Schillberg S, Finnern R. Plant Molecular Farming for the Production of Valuable Proteins: Critical Evaluation of Achievements and Future Challenges. J. Plant Physiol. 258–259, 2021: 153359; https://doi.org/10.1016/j.jplph.2020.153359.
15 Diamos AG, et al. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Front. Bioeng. Biotechnol. 7 2020: 472; https://doi.org/10.3389/fbioe.2019.00472.
16 Swope K, et al. Reproducibility and Flexibility of Monoclonal Antibody Production with Nicotiana benthamiana. mAbs 14(1) 2022: e2013594; https://doi.org/10.1080/19420862.2021.2013594.
17 Wakasa Y, Takaiwa F. The Use of Rice Seeds to Produce Human Pharmaceuticals for Oral Therapy. Biotechnol J. 8(10) 2013: 1133–1143; https://doi.org/10.1002/biot.201300065.
18 Scheller J, et al. Production of Spider Silk Proteins in Tobacco and Potato. Nature Biotechnol. 19, 2001: 573–577; https://doi.org/10.1038/89335.
19 Su J, et al. Low Cost Industrial Production of Coagulation Factor IX Bioencapsulated in Lettuce Cells for Oral Tolerance Induction in Hemophilia B. Biomaterials 70, 2015: 84–93; https://doi.org/10.1016/j.jplph.2020.153359.
20 Meyers B, et al. Nuclear and Plastid Genetic Engineering of Plants: Comparison of Opportunities and Challenges. Biotechnol. Adv. 28, 2010: 747–756; https://doi.org/10.1016/j.biotechadv.2010.05.022.
21 Bally J, et al. The Rise and Rise of Nicotiana benthamiana: A Plant for All Reasons. Annu. Rev. Phytopathol. 56, 2018: 405–426; https://doi.org/10.1146/annurev-phyto-080417-050141.
22 Gelvin SB. Integration of Agrobacterium T-DNA into the Plant Genome. Annu. Rev. Genetics. 51, 2012: 195–217; https://doi.org/10.1146/annurev-genet-120215-035320.
23 Hefferon KL. Plant Virus Expression Vectors Set the Stage As Production Platforms for Biopharmaceutical Proteins. Virology 433, 2012: 1–6; https://doi.org/10.1016/j.virol.2012.06.012.
24 Garabagi F, et al. Utility of the P19 Suppressor of Gene-Silencing Protein for Production of Therapeutic Antibodies in Nicotiana Expression Hosts. Plant Biotechnol. J. 10(9) 2012: 1118–1128; https://doi.org/10.1111/j.1467-7652.2012.00742.x.
25 Laxa M. Intron-Mediated Enhancement: A Tool for Heterologous Gene Expression in Plants? Front Plant Sci. 7, 2017: 1977; https://doi.org/10.3389/fpls.2016.01977.
26 Viegas VSM, Ocampo CG, Petruccelli C. Vacuolar Deposition of Recombinant Proteins in Plant Vegetative Organs As a Strategy to Increase Yield. Bioengineered 8(3) 2017:
203–211; https://doi.org/10.1080/21655979.2016.1222994.
27 Kallolimath S, et al. Promoter Choice Impacts the Efficiency of Plant Glycol-Engineering. Biotechnol J. 13(1) 2018: 1700380; https://doi.org/10.1002/biot.
201700380.
28 Diamos AG, et al. 5′ and 3′ Untranslated Regions Strongly Enhance Performance of Geminiviral Replicons in Nicotiana benthamiana Leaves. Front. Plant Sci. 7, 2016: 200; https://doi.org/10.3389/fpls.2016.00200.
29 Leth KI and McDonald KA. Media Development for Large Scale Agrobacterium tumefaciens Culture. Biotechnol. Prog. 33(5) 2017: 1218–1225; https://doi.org/10.1002/btpr.2504.
30 Prudhomme N, et al. Quantitative Proteomic Profiling of Shake Flask Versus Bioreactor Growth Reveals Distinct Responses of Agrobacterium tumefaciens for Preparation in Molecular Pharming. Can. J. Microbiol. 67(1) 2021: 75–84; https://doi.org/10.1139/cjm-2020-0238.
31 Houdelet M, et al. Animal Component-Free Agrobacterium tumefaciens Cultivation Media for Better GMP Compliance Increases Biomass Yield and Pharmaceutical Protein Expression in Nicotiana benthamiana. Biotechnol. J. 12(4) 2017: https://doi.org/10.1002/biot.201600721.
32 Prudhomme N, et al. Exposure of Agrobacterium tumefaciens to Agroinfiltration Medium Demonstrates Cellular Remodelling and May Promote Enhanced Adaptability for Molecular Pharming. Can. J. Microbiol. 67(1) 2021: 85–97; https://doi.org/10.1139/cjm-2020-0239.
33 Fujiuchi N, Matoba N, Matsuda R. Environment Control to Improve Recombinant Protein Yields in Plants Based on Agrobacterium-Mediated Transient Gene Expression. Front. Bioeng. Biotechnol. 4, 2016: 23; https://doi.org/10.3389/fbioe.2016.
00023.
34 Knödler M, et al. Seasonal Weather Changes Affect the Yield and Quality of Recombinant Proteins Produced in Transgenic Tobacco Plants in a Greenhouse Setting. Front. Plant Sci. 10, 2019: 1245; https://doi.org/10.3389/fpls.2019.01245.
35 Dusek J, et al. Extended Set of GoldenBraid Compatible Vectors for Fast Assembly of Multigenic Constructs and Their Use to Create Geminiviral Expression Vectors. Front. Plant Sci. 11, 2020: 522059; https://doi.org/10.3389/fpls.2020.522059.
36 Irmisch S, et al. Complete Biosynthesis of the Anti-Diabetic Plant Metabolite Montbretin A. Plant Physiol. 184(1) 2020: 97–109; https://doi.org/10.1104/pp.20.00522.
37 Gelvin SB. Traversing the Cell: Agrobacterium T-DNA’s Journey to the Host Genome. Front. Plant Sci. 3, 2012: 52; https://doi.org/10.3389/fpls.2012.00052.
38 Buyel JF, et al. Extraction and Downstream Processing of Plant-Derived Recombinant Proteins. Biotechnol. Adv. 33(6) 2015: 902–913; https://doi.org/10.1016/j.biotechadv.2015.04.010.
39 Rössner C, Lotz D, Becker A. VIGS Goes Viral: How VIGS Transforms Our Understanding of Plant Science. Annu. Rev. Plant Biol. 73, 2022 (in press); https://doi.org/10.1146/annurev-arplant-102820-020542.
40 Yoshida T, et al. Genome Defense Against Integrated Organellar DNBA Fragments from Plastids into Plant Nuclear Genomes Through DNA Methylation. Nature Sci. Rep. 9, 2019: 2060; https://doi.org/10.1038/s41598-019-38607-6.
41 Scholthof HB. The Tombusvirus-Encoded P19: From Irrelevance to Elegance. Nat. Rev. Microbiol. 4(5) 2006: 405–411; https://doi.org/10.1038/nrmicro1395.
42 Jutras PV, Dods I, van der Hoorn RA. Proteases of Nicotiana benthamiana: An Emerging Battle for Molecular Farming. Curr. Opin. Biotechnol. 61, 2020: 60–65; https://doi.org/10.1016/j.copbio.2019.10.006.
43 Bally J, et al. The Extremophile Nicotiana benthamiana Has Traded Viral Defence for Early Vigour. Nature Plants 1, 2015: 15165; https://doi.org/10.1038/nplants.2015.165.
44 Schoberer J, Strasser R. Plant Glycol-Biotechnology. Semin. Cell Dev. Biol. 80, 2018: 133–141; https://doi.org/10.1016/j.semcdb.2017.07.005.
45 Kaulfürst-Sobell H, et al. Reduced Immunogenicity of Arabidopsis hgl1 Mutant N-Glycans Caused by Altered Accessibility of Xylose and Core Fucose Epitopes. J. Biol. Chem. 286(26) 2011: 22955–22964; https://dx.doi.org/10.1074%2Fjbc.M110.196097.
46 Strasser R, et al. Generation of Glyco-Engineered Nicotiana benthamiana for the Production of Monoclonal Antibodies with a Homogeneous Human-Like N-Glycan Structure. Plant Biotechnol. J. 6(4) 2008: 392–402; https://doi.org/10.1111/j.1467-7652.
2008.00330.x.
47 Jansing J, et al. CRISPR/Cas9-Mediated Knockout of Six Glycosyltransferase Genes in Nicotiana benthamiana for the Production of Recombinant Proteins Lacking β-1,2-Xylose and Core α-1,3-Fucose. Plant Biotechnol. J. 17(2) 2019: 350–361; https://doi.org/10.1111/pbi.12981.
48 Schwestka J, et al. Impact of Specific N-Glycan Modifications on the Use of Plant-Produced SARS-CoV-2 Antigens in Serological Assays. Front. Plant Sci. 12, 2021: 747500; https://doi.org/10.3389/fpls.2021.747500.
49 Maharjan PM, et al. Plant-Expressed Receptor Binding Domain of the SARS-CoV-2 Spike Protein Elicits Humoral Immunity in Mice. Vaccines 9(9) 2021: 978; https://doi.org/10.3390/vaccines9090978.
50 Castilho A, et al. Generation of Enzymatically Competent SARS-CoV-2 Decoy Receptor ACE2-Fc in Glycoengineered Nicotiana benthamiana. Biotechnol. J. 16(6) 2021: e2000566; https://doi.org/10.1002/biot.202000566.
Corresponding author Doug Cossar, PhD, is vice president of research; Michael D. McLean, PhD, is director of research; and Don Stewart, PhD, is president and chief executive officer at PlantForm Corporation, 1920 Yonge Street, Suite 200, Toronto, Ontario M4S 3E2; [email protected]; 1-416-572-7795.
You May Also Like






