Insulin in Demand: Engineering a Facility to Serve Local and International MarketsInsulin in Demand: Engineering a Facility to Serve Local and International Markets
Insulin injection
Discovered in 1922 at the University of Toronto (by Frederick Banting and John James Rickard Macleod), insulin has been produced from animal extract, primarily cattle and pigs. Following Frederick Sanger’s work on insulin sequence in 1951, Dorothy Hogkin published in 1969 the three-dimensional structure of insulin. This breakthrough led to many developments and applications of recombinant DNA for the production of insulin, human first and then analogs.
The significant increase in the diabetic population — especially in BRICS countries (Brazil, Russia, India, China, and South Africa) — has pushed local pharmaceutical manufacturers to focus on this major health issue. These nations are developing either local production capacities or an adequate supply chain to answer domestic demands. But insulin production can bring many difficulties and requires robust processes to ensure the safety and effectiveness of final products.
Herein we discuss how the engineering company Biopharmax established an insulin plant in China in a turnkey project model, supported by Novasep as the chromatography systems provider. The facility was designed to comply with requirements for both local and international markets.
Prevalence of Diabetes in BRICS Population’s
Since insulin’s discovery, its production has become a strategic therapeutic application due to the unfortunate increase of diabetes prevalence among the world’s population. In 2014, the number of people suffering from diabetes was estimated at 387 million (1).
Currently, the demand for insulin treatments largely exceeds worldwide manufacturing capacities, especially in the BRICS nations, where diabetes is recognized as a major health concern by local governments.
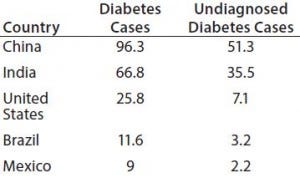
Table 1: Five countries where the number (in millions) of people ages 20–79 who are living with diabetes was the highest in 2014 (1)
China’s and India’s diabetic populations rank among the most prevalent at 25% and 17%, respectively, of the total number of people living with the disease (Table 1). And the Russian Federation has recorded 23% of deaths caused by diabetes. The number of diabetic people is expected to grow up to 592 million in 2035 (a 53% increase), especially in Asia and South America.
Challenges for BRICS Nations in Producing Insulin
The domestic demands of BRICS countries clearly require the development of local insulin manufacturing and secure local supply chains. Such trends in local production are rising, especially since the expiration of multiple patents in 2014–2015, concurrent development of biosimilars, and need for lower-priced biologics.
China already has stepped into the pharmaceutical market (US$75 billion, third biggest after US and Japan) and is currently the world’s largest national biosimilars producer (2). The number of Indian pharmaceutical manufacturers also is growing. Latin America’s domestic production market, although smaller than China’s and India’s, is growing, as reported in the report Top 25 Biosimilar Drug Manufacturers (3).
All production guidelines and compliance requirements are not the same. They depend on the targeted market. A facility that does is not certified as current good manufacturing practice (CGMP) compliant will be systematically disqualified from selling products in the United States or European Union. It is of strategic importance for BRICS manufacturers to comply with international quality standards and increase their competitiveness to market in domestic and international regions.
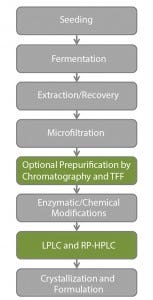
Figure 1: Industrial steps for insulin production
Key Steps for Industrial Production of Insulin
Although insulin production is a well-known process and manufacturing can be generalized (Figure 1), modern production and purification processes and technologies remain confidential among highly competitive producers.
Insulin manufacturing can be complicated. It involves a sophisticated multistep process from fermentation to formulation through a large number of purification steps. All insulin production and purification steps can potentially introduce heterogeneities that can influence biological and clinical properties of a final product.
One key element of recombinant insulin production is the choice of an adequate expression system, which can directly influence the purification in downstream processing in general. The main expression systems are the bacteria Escherichia coli and the yeasts Saccharomyces cerevisiae and Pichia pastoris. These systems generate different challenges and impurity profiles. For example, yeast systems, production in bacteria usually requires a lysis and protein refolding in diluted conditions. So bacteria systems require the implementation of chromatography and/or tangential-flow filtration (TFF)
prepurification steps.
An enzymatic cleavage is often required in both systems to form mature insulin from proinsulin. And final purification is achieved by combining at least two orthogonal chromatography techniques, usually based on ion exchange and reverse-phase chromatography for polishing.
Depending on whether a final product is meant to be rapid or long acting, the amino-acid sequence may be modified to produce different insulin analogs. The physicochemical properties of each analog are different from one another, thereby requiring additional adaptation of purification steps.
In addition, a manufacturing line has to be systematically adapted to the producer’s end markets and must comply with associated regulatory constraints. Important technological differences can depend on targeted specifications. Typically, size-exclusion chromatography is not used in the same way for all manufacturers, and chromatography resins can differ from producers in Asia to South America.
Chromatography is the critical technology for insulin purification, with two to three orthogonal chromatography steps, regardless of the production system. In addition, true process development expertise is important to adapt equipment to product specificities. Eventually, the complexities and variations among production lines also raise challenges of integrating the right technologies into coherent and efficient process lines.
Challenges of Technological Integration
A local company in China looking to manufacture insulin for the local market entrusted Biopharmax to establish a turnkey manufacturing plant, including design, construction, commissioning, and with US Food and Drug Administration (USFDA), European Medicines Agency (EMEA), and China Food and Drug Administration (CFDA) regulatory requirements. Because the customer had a strong business orientation, with little operational experience with insulin manufacturing, Biopharmax took the main responsibility for selecting equipment and implementing a technology transfer of a suitable recombinant E. coli cell line from a third party.
The project’s overall scope included whole-plant design; equipment selection, procurement, integration, and start-up; cleaning systems and plant utilities; heating, ventilation, and air conditioning (HVAC) and cleanrooms; and laboratories and a R&D center. Systems were installed and validated in accordance with the International Society for Pharmaceutical Engineering (ISPE) Pharmaceutical Engineering Guide, Volume 5: Commissioning and Qualification.
Fermentation was a great, complex engineering challenge. Scale-up is usually made at a scale of 1:50, and implies changes in material behavior such as decrease of separation performance and loss of material in a process (which can affect yield). The scale-up in this project was a success —from 100 L to 5,000 L (1:50) without quality loss.
A project of this magnitude relied on more than 250 subcontractors and suppliers from a wide range of disciplines. Working in an international environment with suppliers from all over the world required a well-defined project management strategy to bridge over cultures, different practices, and language barriers. Choosing skilled workforces and educating them in the specifications of international standards to sustain regulatory compliance was successful. Local support and effective communication from vendors were crucial for success.

Photo 1: Chromatography automation system
A fully automated plant and production processes were implemented to simplify human tasks and reduce human intervention. To achieve these goals, a highly sophisticated automation system was developed with >10,000 input/outputs (I/Os) and eight programmable logic controls (PLCs). The batch control program includes >50 recipes and hundreds of phases, compliant with ANSI/ISA-88 standards. A completely integrative control system enables control of all end-point systems from the central control unit. The system was implemented in accordance with 21 CFR Part 11, and GAMP 5 ISPE guidelines for the validation of automated systems in pharmaceutical manufacture.
Meeting such challenges meant that the Biopharmax engineering team had to work closely with technology providers to understand the delicate nuances of the process. Providers were selected based on the quality of their equipment (especially for critical operation units) and on their expertise for process optimization and equipment integration into the process line. This approach resulted in an overall optimization of process equipment, promising high yields and reduced operational costs.
Novasep was chosen for the entire chromatography system including high-performance liquid chromatography (HPLC) system (Prochrom) and low-pressure liquid chromatography (LPLC ) equipment (Prochrom Bio). The Dynamic Axial Compression concept introduced by Novasep for its HPLC chromatography system, and the Hydraulically Actuated Piston concept for LPLC systems helpful in meeting all those needs. They helped to reduce dependency on operators’ skills, optimize cycle time, and make resin replacement activities simpler and more efficient in a relatively shorter time.
Novasep also has an important technical and operational presence in China. So it can offer support to local teams for commissioning, qualification, and trainings as well as for first purification runs.
“After handing over more than 100 projects worldwide, we have now established a very capable and dedicated local team of engineers in China that can continuously serve local market and has even assisted Biopharmax efforts overseas with professional advice and supervision” states Elad Mark, head of engineering at Biopharmax.

Photo 2: Novasep high-performance liquid chromatography column
Choose the Right Chromatography Workhorse
Insulin manufacturing typically involves large volumes of material to treat and purify, which requires industrial systems that can sustain 24/7 operations. Scalability and robustness of such systems must be demonstrated in their suppliers’ track records and those suppliers’ expertise in developing large-scale systems for insulin purification.
Cleaning operations also can greatly affect productivity and overall performance of chromatography lines. Insulin is known to accumulate in chromatography resin and in frits, decreasing the efficiency of a purification process after a few weeks in operation. So manufacturers must regularly clean and unpack HPLC columns (more often than for most of other applications).
To disassemble component parts following purification runs, traditional LPLC columns require hoists, service frames, and safety supports, which are costly, cumbersome, and potentially dangerous. At industrial scale, columns with a motorized piston can help operators perform this operation quickly and efficiently. Using those columns also makes required maintenance tasks easier.
Novasep Prochrom and Prochrom Bio columns are designed with handling solutions so that operators have easy access at floor level to column parts, gaskets, and frits. Dismantling and cleaning a column is no longer a challenge.

Photo 3: Novasep high-performance liquid chromatography column
The Prochrom and Prochrom Bio columns also eliminate the need for bulky lifting equipment, so operators don’t need to handle heavy column components, which speeds up maintenance and reduces risk of worker injury or damage to column tubes, column parts, and seals. The system also enhances clean-room operations by removing contamination sources from overhead lifting equipment, reducing ceiling height requirements and operational footprints.
Column motorization also affects the homogeneity of a packed chromatography bed, one of the key parameters of purification processes. Achieving good packing quality with low-pressure chromatography systems requires efficient packing methods, which differ strongly from one technology to another. A packing method should primary produce the highest number of plates together with an excellent asymmetry factor. Beyond those numbers, the effect of the packing procedure on daily operations should also be taken into account.
Traditional LPLC systems leave no choice to a user but to fully unpack and then repack the column when separation performances are decreasing. Unpacking and repacking operations can take 24 to 48 hours for large-scale columns (>800 mm I.D.) and carry a risk of losing and/or damaging part of the resin. Most of the time, users will not repack a column and will operate it at a lower efficiency, thereby reducing yield and quality. Novasep Prochrom Bio columns allow users to repack the column within a few minutes and give users the opportunity to test several packing conditions to reach the best packing quality.
Resin developers continue to introduce new high-performance media. Especially for low-pressure chromatography steps, these modern, sophisticated resins can be operated at higher velocities. That leads to higher productivities and generates higher pressure drops. Whereas most LPLC columns are rated at 3 bar, 5-bar Prochrom Bio columns allow the use of advanced resin technologies.
Polishing is a Work of Art
Insulin manufacturing generates impurities very similar to insulin itself both in structural and chromatographic behavior. As a result, such impurities can be difficult to eliminate. Because elution peaks of insulin and “pseudo-insulin impurities” are very close, excellent resolution is required. Good resolution results from the combination of a high number of theoretical plates and excellent selectivity. Reverse-phase HPLC is the most efficient technique to recover pure insulin, but only parameter optimization can ensure high yields.
Technological excellence is a central element for quick, efficient, and robust chromatography purification. Process development expertise is another factor. Understanding the whole insulin process facilitates not only efficient introduction of purification equipment in that process, but also the training of local teams. Close support — consisting of operator pretraining and strong technical assistance — helped final users reach constant performance in each batch, with results that were comparable with the pilot test. Four months after start-up, a second and final training session specifically focused on unpacking and repacking the columns brought complete autonomy to the operators.
A Successful Approach
A true understanding of the insulin purification process maximizes equipment performance and enhances competitiveness. Finding partners with process development skills and the ability to support manufacturers facing their own technical challenges is a real advantage that should always be considered.
Close coordination with equipment manufacturers is a proven success strategy. Results of the first trial batch produced demonstrated outstanding yields with a purity of final product exceeding 99%. “Being able to provide the client with a state-of-the-art facility, yielding production at the highest possible standards, and with proven commercial efficiency unquestionably determines this as a flagship project,” says Mark.
References
1 IDF Diabetes Atlas, Sixth edition, 2014 Update, International Diabetes Federation: Brussels, Belgium. ISBN: 2-930229-85-3; www.idf.org/diabetesatlas.
2 Biosimilars in China: The Coming Revolution; BioPharmaDive 22 October 2014; www.biopharmadive.com/news/best-of-bd-biosimilars-in-china-the-coming-revolution/324525.
3 Top 25 Biosimilar Drug Manufacturers 2012–2022. Visiongain: London, England, 10 January 2012; www.visiongain.com/Report/906/Pharma-Leader-Series-Top-25-Biosimilar-Drug-Manufacturers-2012-2022.
Corresponding author Solomon Gahtan is vice president of business development at Biopharmax Group Ltd., 4 Hasadnaot Street, PO Box 12644, Herzelia Pituch 46728, Israel; 972-9-9716111/165; [email protected]. Primael Fablet is area manager (Asia) at Novasep, and Nicolas-Julian Hilbold was R&D and pilot engineer at Novasep.
You May Also Like






