Next-Generation Laboratory Moves: Strategies To Maximize Your Laboratory-Relocation PlanNext-Generation Laboratory Moves: Strategies To Maximize Your Laboratory-Relocation Plan
October 15, 2024
Laboratory and facility relocations often signal growth and progress for organizations. However, many lack the internal technical and relocation expertise necessary for a seamless move, which is crucial to minimizing downtime and preventing disruptions to daily operations and research activities. That situation underscores the importance of engaging experienced relocation engineers and technical project managers whose expertise can enhance operational efficiency significantly during transitions to new laboratory facilities. Such experts have provided support to hundreds of life-science organizations by developing and executing their laboratory-relocation plans. Whether driven by capacity constraints, the need for expanded research activities, and/or compliance demands, such experts can give guidance through every phase of the process. Herein, I explore key strategies for maximizing efficiency within a laboratory-relocation plan.
Establishing a Comprehensive, Phased Move Plan
When an organization is ready to invest in a new laboratory facility to advance its growth objectives, it first must develop a comprehensive move plan. Using a phased approach and initiating the planning process early are critical steps in minimizing operational disruptions and enabling a well-synchronized relocation process for your laboratory (1).
Facilities must collaborate with clients to develop relocation plans tailored to their laboratories’ operational schedules and construction timelines. Carefully strategizing optimal sequences for relocating personnel, equipment, and other critical materials ensures maintained productivity and preserved research continuity (Figure 1). Assembling a cross-functional relocation team of essential stakeholders — e.g., facility and project management, operations, external move vendors, and key subject-matter experts (SMEs) for calibration; commissioning, qualification, and validation (CQV); quality; and regulatory affairs — is also essential (2). Engaging those stakeholders early in the planning process enables an organization to develop a comprehensive and well-informed relocation plan.

Figure 1: Overview of a core relocation team.
Relocation-Team Structure: As companies progress through their development life cycles, the complexity of laboratory relocations tends to increase as more stringent requirements are applied. Safety, regulatory compliance, and management processes for equipment, inventory, and data are critical aspects of a laboratory’s phased move plan.
Key Requirements and Considerations: Ensuring safety compliance can entail following regulations that concern the transportation of hazardous materials, chemicals, and biological samples. Transport protocols should account for proper packaging, labeling, and documentation of such materials. Regulatory compliance can encompass meeting guidelines — such as the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and standards from the College of American Pathologists (CAP) — as well as good laboratory practices (GLPs) and/or good manufacturing practices (GMPs). Compliance can necessitate permits, licenses, certifications, and/or outside inspections.
Additionally, relocation teams must consider the scope and complexity of moving sensitive laboratory equipment and instrumentation, accounting for factors including size, weight, fragility, calibration needs, and specialized handling requirements. Assessing the inventory of laboratory supplies, consumables, reagents, and samples is essential to determine relocation priorities and ensure appropriate handling and storage of such materials. Furthermore, relocation teams must prioritize the secure transfer and backup of electronic data, research records, and laboratory-information management systems (LIMs) to uphold data integrity and accessibility throughout the relocation process. Collaboration with relevant functional owners is critical for defining those requirements and integrating them into a phased move plan (Figure 2).
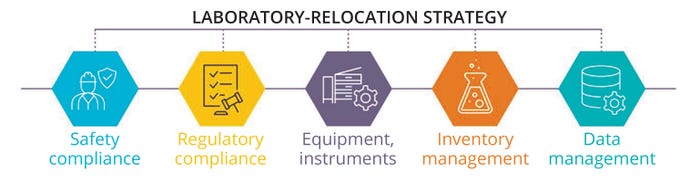
Figure 2: Aspects to consider in a laboratory-relocation strategy.
Choosing the Right Move Partner: Another critical factor for life-science organizations to consider in their relocation strategy is the selection of the right moving partner. Such partners must be equipped with the necessary expertise and resources to relocate sensitive laboratory equipment and materials effectively (3). Before embarking on the laboratory-relocation process, you must conduct research to inform your move-vendor selection and to help your relocation team establish appropriate oversight. Steps for selecting the right moving partner include conducting thorough online research, seeking referrals and recommendations from reliable colleagues and other vendors, and subsequently compiling a list of candidates. Engaging in in-person walkthroughs and discussions with potential vendors can help you to evaluate their capabilities and experience, ensuring that they can effectively meet the specific requirements of your laboratory relocation.
Criteria for choosing a suitable move partner involve extensive experience in laboratory and facility relocations as well as ensuring that they possess the expertise to navigate the intricacies of a move (Figure 3). Look for partners with a proven track record in handling sensitive laboratory equipment, cold-chain logistics, and the flexibility to accommodate tight turnarounds. Check into whether a move partner can provide a comprehensive service offering from preparation to post-move support. Additionally, a commitment to clear communication and a positive culture further validate a vendor’s suitability.

Figure 3: Steps for selecting a relocation vendor.
A suitable partner will offer a realistic cost estimate, provide positive references from previous customers, identify and address distinctive challenges up front, and demonstrate their ability to deliver a successful relocation within the required time frame. Leveraging extensive experience in laboratory relocations supports clients in selecting service providers that align with their unique requirements. Doing so ensures a confident execution of the laboratory move, supported by a professional team that is skilled in navigating the complexities of such projects.
Equipment Mapping and Process Optimization: During laboratory relocation, the primary objective is to minimize downtime, which requires a carefully crafted strategy. That requires mapping equipment layout in the new facility and scheduling necessary maintenance, calibration, and qualification to ensure that assets are returned to use promptly.
Effective equipment mapping requires collaboration among the relocation team and representatives from different functional groups, including quality, laboratory operations, information technology (IT), facilities, and engineering. By carefully mapping the placement of each piece of equipment, laboratories can ensure adequate space and utility availability, enabling a swift return to operations after the move. Additionally, the use of technologies such as computer-aided design (CAD) software can facilitate mapping processes further for the new space. Once the equipment is placed in its final destination, the phased move plan becomes invaluable, allowing for immediate scheduling of equipment calibrations, qualifications, and other preventative and operational maintenance required for each asset. Such a proactive approach ensures that all instruments adhere to industry standards and are ready for use promptly upon arrival at the new facility, thereby sustaining productivity and minimizing any disruption to operations.
By leveraging comprehensive solutions in effective equipment mapping, including the application of operational excellence principles and essential software tools, laboratories can achieve seamless transitions, maintain productivity, and minimize disruptions during relocations. The incorporation of 5S principles, shown in Figure 4, ensures that the new facility is organized efficiently from the start (4, 5). That methodical approach can help facilities to maintain clean, orderly, and efficient workspaces, which support sustained productivity and operational excellence throughout and after the move.

Figure 4: 5S principles for facility relocation.
Such a holistic strategy establishes a foundation for efficiency and continuous improvement throughout the relocation process. This approach combines technical proficiency and commitment to operational excellence. A cross-functional team, which should include engineers, project managers, and experts in six sigma and lean methodologies, is dedicated to maximizing the value of clients’ relocation investments. By leveraging collective expertise, the team can ensure heightened efficiency and productivity in the new facility, empowering clients to achieve optimal business outcomes.
Results of a Well-Executed Relocation Plan
A robust relocation plan should minimize operational downtime and set up optimal performance in the new facility. Operational excellence is crucial in preparing laboratories for those transitions, emphasizing continuous improvement and process optimization within the relocation plan. A strong relocation strategy should include tangible measures for your organization such as enhanced efficiency, reduced operational expenses, and improved business outcomes for the future.
References
1 Dotzert M. Laboratory Relocation in Three Stages. Lab Manager, 4 July 2019; https://www.labmanager.com/laboratory-relocation-in-three-stages-918.
2 Laboratory Facility Construction and Major Renovations Guidelines. Association of Public Health Laboratories: Silver Spring, MD, 2019; https://www.aphl.org/aboutAPHL/publications/Documents/GH-2019May-Lab-Construction-Reno-Guidelines.pdf.
3 Dance G. Partnering for a Successful Lab Relocation. American Laboratory, 4 February 2022; https://www.americanlaboratory.com/583451-Partnering-for-a-Successful-Lab-Relocation/.
4 McDevitt K. The Benefits of Embedding Safety in Lean Practices. BioProcess Int. 12(5) 2014: 32–37; https://www.bioprocessintl.com/analytical/the-benefits-of-embedding-safety-in-lean-practices.
5 Sandle T, Vanderstel G, Huynh-Ba K. BPI Lab — Contemporary Approaches to Data and Organization. eBook. BioProcess Int. 18 October 2022; https://www.bioprocessintl.com/laboratory-equipment/ebook-bpi-lab-contemporary-approaches-to-data-and-organization.
Jasmina Xie is a senior project manager and construction manager at Sequoia Biotech Consulting; 315 South Coast Highway 101, Suite U58, Encinitas, CA 92024, [email protected].
You May Also Like






