A Holistic Approach: Bridging the Gap Between Suppliers and BiomanufacturersA Holistic Approach: Bridging the Gap Between Suppliers and Biomanufacturers
September 30, 2021
 How can biopharmaceutical manufacturers expect their suppliers to deliver what the industry wants and needs if it doesn’t communicate those desires? Without industry input, biotechnology suppliers are developing technologies “blindly.” Despite their having delivered great value over the years, together the greater community can do better. Aligned industry input is a vital element in the development process. Here we describe how two BioPhorum workstreams focused on drug-product development have worked to facilitate these communications.
How can biopharmaceutical manufacturers expect their suppliers to deliver what the industry wants and needs if it doesn’t communicate those desires? Without industry input, biotechnology suppliers are developing technologies “blindly.” Despite their having delivered great value over the years, together the greater community can do better. Aligned industry input is a vital element in the development process. Here we describe how two BioPhorum workstreams focused on drug-product development have worked to facilitate these communications.
Last summer, the container–closure integrity (CCI) workstream gave advance notice in a BPI eBook of its planned suite of papers describing how a quality- and risk-based holistic approach could remove the need for routine CCI testing (1). Keeping unnecessary testing burdens to a minimum enhances manufacturing efficiencies and maintains patient supplies through building quality into products and processes — rather than fixating on a “testing into compliance” mindset.
The holistic approach to CCI covers how to build process knowledge and understanding (while reducing/removing CCI testing) across the life cycle of a biopharmaceutical product. With five sections from initial container–closer system (CCS) development through qualification, validation, routine manufacturing, and ultimately shipping and stability testing, the approach seeks to describe key aspects at each stage of that life cycle that feed into and build a holistic approach.
Those five stages are largely complete now. The final stage of the paper’s development was to build a common thread throughout to illustrate how a holistic approach could be realized. Prefilled syringes will serve as an example of building a holistic approach for one type of biopharmaceutical product presentation. Different methodologies will be highlighted in tables where (they are) appropriate for different presentation types, making clear where similarities and differences in approach are needed. This will be the final piece of the workstream’s holistic-approach development before the team begins its internal review and approval process.
The holistic approach is meant as a comprehensive resource for companies wishing to demonstrate a robust understanding of their products and processes underpinned with robust quality-management systems. Figure 1 provides an overall view of the holistic approach, with three potential outputs: no routine CCI testing, some mandated CCI testing, and full container–closure integrity testing (CCIT) required (see box, below).
The overview diagram identifies two needs extending from the middle-ground scenario. To perform CCIT on a given product, that product first must be sampled and then tested — necessitating a documented sampling approach and a qualified test method. To address those two needs, the CCI workstream recently published a paper on alternative sampling methods and a user requirement specification (URS) focused on the biopharmaceutical industry’s container–closure integrity testing technology needs (2–3).
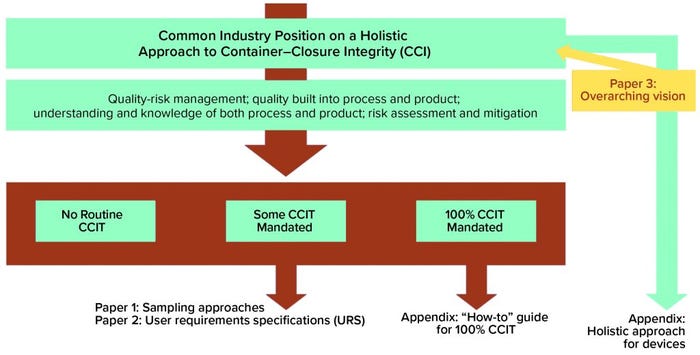
Figure 1: The holistic approach to container–closure integrity testing (CCIT)
Sampling
When sampling is required for container–closure integrity testing, scientifically valid approaches to it should be based on risk assessments and information such as supplier approval, packaging component specifications, and process knowledge. CCIT sampling approaches differ across the industry, with no official standard or regulatory guidance for sampling or definitions of appropriate sample sizes. That can be concerning for destructive test methods because sample numbers and time required for testing can endanger the continuity of patient supply. BioPhorum’s sampling paper addresses those gaps, providing options for defining a justifiable sample size for container–closure integrity testing and summarizing the options for risk-based strategies. That should help readers consider their options for demonstrating a scientific justification for their chosen approach.
The paper illustrates how to eliminate unnecessary sampling and testing without affecting product quality. Increased sample sizes often are considered to be necessary for larger batch sizes, but sampling based on the risk level of defects (and used in conjunction with common sampling plans) is a more appropriate approach.
We do not prescribe a one-size-fits-all approach, but rather offer some concepts for scientifically justified sampling plans that do not add unnecessary testing burden (when applied within a robust quality-management strategy). Alternative approaches might be applied if a biopharmaceutical company needs to sample and test drug product lots for CCI. Particularly for the smaller sizes expected for new products and presentations, our risk-based strategy facilitates faster testing where required, without adversely affecting patient supply.
Container–Closure Integrity Testing (CCIT) Paradigms |
|---|
No Routine CCIT Required: This is perhaps an ideal scenario in which all processes are fully understood. A company maintains a robust quality risk management (QRM) approach, and all risks are assessed, with mitigation approaches in place. Some CCIT Mandated: At least for the short-term, this is the most likely scenario. Process knowledge and understanding are high, although some risks remain less well understood or are not fully mitigated, which makes some CCIT necessary for confirmation of integrity. 100% CCIT Mandated: For processes that are difficult to understand or predict, or where some risks are either unknown or difficult to mitigate, CCIT will be necessary throughout the life cycle. |
Container–Closure Integrity Testing Technologies
The CCIT workstream’s late-2020 URS paper is another key element of the broader holistic approach. It highlights a long-term desire for equipment that can better meet end-user requirements. As more vendors develop equipment to meet these requirements, the industry will gain confidence that CCIT methods can demonstrate container integrity effectively.
The URS provides a long-term goal for companies that often use multiple CCIT technologies with different capabilities to support the requirements of expanding product portfolios. The paper will help developers overcome significant inefficiencies to resolve the problem that deterministic CCIT methods currently available do not fulfill all CCIT needs.
Subject matter experts (SMEs) in the CCI workstream are keen to leverage publication of our URS to engage with suppliers. After some initial contact online, open conversations have kicked off tentatively this summer. Now we are extending an invitation for all CCIT developers, suppliers, and stakeholders to contribute. If the team hasn’t reached out to you directly, that is simply a consequence of bandwidth. We encourage you to get in touch with the CCI SMEs to keep the conversation as inclusive as possible.
Although we believe that all of the elements of our URS are important to the future of container–closure integrity testing, the workstream members do recognize that for technology developers to work on everything at once would be impractical. We have been working hard to prioritize key elements from our URS to help vendors focus their attention on what could be the first step in industry-supported development of CCIT technologies. Within the workstream, we hope that these discussions will develop into a longer-term partnership between an aligned industry group and CCIT vendors, all working together to develop technologies that meet the needs of the industry and truly ensure CCI for the future.
Next Steps
As the team approaches completion of its holistic approach to CCI, the members are acutely aware that this is not the end of the story. The workstream already has planned two further publications that will complete the overarching vision.
100% CCIT: The next step to complete the continuum of testing needs arising from a risk-based approach concerns — the ideal state being one in which no CCIT is required; the flip-side of that coin is a scenario in which 100% is required. The first appendix to the holistic approach will explore what types of situations drive the need for 100% testing. It will describe how a 100% CCIT system would be qualified, validated, used, and maintained. It should address questions related to data management, use, trending, and so on. The team is already discussing those questions, with some members successfully running 100% CCIT on lyophilized products with modified headspaces. However, not all products and test methods lend themselves to 100% CCIT, so we will attempt to address such harder-to-answer questions as we progress.
Delivery Devices: The second appendix to the holistic approach will examine at a high level how to apply it to delivery devices. From prefilled syringes to multidose combination devices, BioPhorum members are witnessing an expansion of biopharmaceutical products presented in complex containers. CCIT traditionally has proven to be difficult to apply to such devices, and uncertainties abound with a paucity of guidance covering them. So the CCI workstream hopes to provide some clarity and guidance in this space.
An Invitation: In addition to paving the way toward a more quality- and risk-based approach to understanding container and product integrity, the holistic approach and URS represent an opportunity to drive progress on how CCI is assured and how CCIT is performed.
Biomanufacturers have identified the needs of their industry for such technologies. These publications represent an invitation to the CCIT supplier community. Users are eager to engage with their suppliers to work toward shaping solutions that will benefit not just the industry, but ultimately, patients.

Groninger SmartFill system (https://www.groninger.de)
Bridging the Gap: A Case Study
To illustrate the value of industry-driven URS approaches, a recent success story makes the case for collaborations between users and suppliers — moving away from CCI to an entirely different aspect of biopharmaceutical drug-product manufacturing. Without direction from their customers (pharmaceutical manufacturers of all sizes), filling- machine and isolator vendors work “in the dark” to develop new approaches and technologies. BioPhorum is reducing the distance between vendors and industry by providing a conduit for collaboration and resources to encourage sharing.
In May 2018, the workstream focused on small flexible fillers published a URS outlining the biopharmaceutical industry’s needs for future development of such fillers (4). It lays the foundations of the industry’s expectations to help its suppliers develop appropriate technologies and focus on the concerns that are most important to biomanufacturers. The URS is intended to help vendors understand what innovations are most needed to facilitate the transition into a world of personalized medicines developed for smaller patient populations.
Shortly after publication of the URS, German-based technology and equipment supplier Groninger began to deliver on its requirements. Within 2.5 years, the company had developed a commercially viable automated filling line that uses robot handling to minimize human interventions. The modular design confers flexibility for filling multiple product formats and toxicities on the same system.
Whereas traditional large-batch manufacturing uses high-speed filling lines to deliver low per-unit costs, the more flexible approach requires agile filling technologies. Filling lines need to accommodate multiple lower-volume products by facilitating rapid changeover to maximize flexibility and capacity. With the concurrent trend toward implementing single-use technologies and components across drug manufacture, the URS opens the door to those flexible approaches while recognizing current limitations of sensors and other technologies.
Meanwhile, tightening sterility restrictions drive the need to move operators farther away from filling processes and reduce the number of human interventions during processing — ideally to none. The URS reflects an expectation that most filling operations ultimately will be automated.
For those reasons, modularity is expected to play a major role in future filling operations. Today’s highly customized, single-product systems are likely to be replaced with simpler, modular systems providing the flexibility to accommodate different products and facilitate changeover between them. The biopharmaceutical industry will see a range of new requirements for small, flexible fillers: e.g., modular plug-and-play capability; gloveless isolators for enhanced sterility assurance; low rejection rates; rapid changeover times; standardized and ready-to-use components; and minimal (ideally no) human intervention.
Before the URS was published, Groninger had its own ideas but needed industry corroboration to find answers to specific questions such what defines a small batch and what counts as “low speed.” The URS gave clear guidance to fill those knowledge gaps. With its specific questions answered, the company could move forward.
Most filler design is fulfilled through bespoke orders, an approach that does not lend itself to shortening lead times or standardizing components. Using the URS to align with the needs of industry provides technology vendors with confidence that a less tailored and more standardized approach could add value through speeding up implementation, reducing inventory, and facilitating rapid response.
Top Priorities: Groninger’s initial development focused on aspects of the URS in which the company could make an immediate impact. That broadly matched the areas that SMEs in the workstream had initially highlighted as most urgent:
fully integrated and automated in-process control (IPC) for fill weight based on feedback
minimal product loss during filling
capability to fill multiple formats with rapid changeover
operation without the need for gloves
ability to handle toxic and nontoxic materials
automated environmental control and monitoring.
Working to those priorities, Groninger initiated a project to create a new isolator line that goes “beyond gloveless robotic fill and finish.” The company planned to move from receiving the URS to incorporating 90% of its elements into a commercially available filling line in 2.5 years. Leveraging automation as a general industry trend, with the focus provided by the URS, Groninger could apply robotics to the need for a gloveless (and ideally, operator-free) filling operation.
The company tackled each priority area with a highly modular approach. Solutions to each challenge were developed individually, but in a rational and connected way that enabled their collation into a series of unit operations, ultimately forming an interconnected and automated filling line. This approach drove development of novel technologies and approaches to meet needs outlined in the URS.
For example, Groninger’s SmartFill system fills liquid product into a primary container while managing priming and redosing with continuous feedback to the pump that ensures even and accurate filling. Intelligent use of robotics prevents air turbulence above open containers, and a “run-empty” mode allows even the final few containers to be filled efficiently, thus minimizing product loss. The line uses automated sorting for all movements, representing a move toward eliminating airborne contamination and addressing many concerns about cleaning, sterilizing, and installation of sorting systems. Designed for a continuous infeed and outfeed of packaging material, product, and waste, the robotic line is not limited by batch size. Robots manage line setup and operations that traditionally have been manual, effectively removing human interventions from the filling process. Using different isolator modules creates distinct zones within the filling system, handling both toxic and nontoxic materials with vacuum-free movements and automated cleaning to enable rapid switching from one product type to the next. Groninger automated environmental monitoring by determining where operator activities could be removed. All infeed and handling of settle plates is automated, with moving parts located below the plates to minimize contamination risk.
The pharmaceutical industry is deliberative and often slow to change, partly because of regulatory requirements. It can be difficult to demonstrate that a new technology provides equivalent or better results than those from existing systems, especially if your company is first to adopt. Groninger secured its first order for the new filling line from a regional contract development and manufacturing company in Germany. By partnering with that customer early in the development process, the supplier benefited from more open communication than is common with multinational customers, providing for rapid feedback and exchange of ideas.
Continued Dialogue: In development of the robotic filler, Groninger made some assumptions to for the sake of time. Industry clients always have specific requirements that will necessitate further development and refinement in working toward creating a perfect filler. This opens the door to maintaining open, transparent dialogue between technology developers and users to ensure that the most important elements of filler design and function are addressed.
Groninger started work on its automated filling line within months of BioPhorum’s publishing its URS for small flexible-filler technology. Within 2.5 years, the supplier had successfully developed a commercially viable line using robot handling to minimize human interventions and a modular design to confer flexibility. In addition to meeting the requirements laid out in the URS, Groninger ensured that the new filler complies with current regulatory expectations.
A Collaborative Future
Such rapid development in response to an aligned industry URS exemplifies how collaborative organizations like BioPhorum can spark the supplier community into direct and specific action to address the needs of the biopharmaceutical industry through positive and lasting change. Groninger’s new automated filling line might not exist in its current format without that published URS.
As BioPhorum members continue to generate industry-aligned URS documents across a range of topics within the fill–finish space and beyond, future technology developments will arise from those discussions. By providing insights into the biopharmaceutical industry’s aligned needs, this organization provides a conduit to bring together biomanufacturers, suppliers, and other key stakeholders for open discussion about designing the future together for the benefit of all.
References
1 Ewan S. A Holistic Approach to Container–Closure Integrity: An Industry-Wide Collaboration. BioProcess Int. eBook June 2020: 6–14; https://bioprocessintl.com/manufacturing/fill-finish/ebook-formulation-fill-finish-conundrum%e2%81%a0-biopharmaceutical-drug-products-for-a-modern-age.
2 Ewan S, et al. Sample Sizing Approaches for Container Closure Integrity (CCI) Testing. Am. Pharm. Rev. 25 February 2021; http://www.americanpharmaceuticalreview.com/Featured-Articles/573671-Sample-Sizing-Approaches-for-Container-Closure-Integrity-CCI-Testing.
3 Ewan S, et al. Container Closure Integrity: User Requirements Specification (URS) for Optimized Container Closure Integrity Testing Equipment. BioPhorum: London, UK, 18 November 2020; https://www.biophorum.com/download/ccit-user-requirements-specification-urs-for-optimized-container-closure-integrity-testing-equipment.
4 Durrin J, et al. User Requirement Specification for Small Flexible Fillers. BioPhorum: London, UK, 17 May 2018: https://www.biophorum.com/wp-content/uploads/bp_downloads/URS-for-SFF-May-2018.pdf.
Consultant Scott Ewan is both director of Ewanique Ltd. and a BioPhorum facilitator based in The Gridiron Building, 1 Pancras Square, London N1C 4AG, UK; [email protected]; https://www.biophorum.com.
You May Also Like






