Balancing Act: A Pathway to Microneedle Manufacturing OptimizationBalancing Act: A Pathway to Microneedle Manufacturing Optimization
November 14, 2024
Microneedle-array patches (MAPs) represent a promising frontier in drug-delivery technology that offers a potential revolution in the administration of medications and vaccines. The technology could combine the ease of transdermal patches with the efficacy of injections, potentially offering more intuitive drug delivery for a wide range of therapeutics (1).
Despite the promising potential of microneedle delivery, several challenges hinder the technology’s development and widespread market acceptance. One primary issue is the limited amount of drug that can be delivered in a single application (2). For many therapeutics, consistent delivery of sufficiently large doses is essential for efficacy. So the limitation has restricted the scope of suitable drug candidates for MAPs primarily to very potent substances, such as hormones and vaccines. Overcoming the challenge of consistent large-dose delivery in a single application could expand the utility of MAP technology, broadening the scope of data available to back up the efficacy of such products.
Another critical challenge in MAP development is the translation of innovative designs into scalable manufacturing processes. As biotechnology companies make strides in early stage development and preclinical studies, researchers need to consider how those advancements will be brought into good manufacturing practice (GMP) clinical production and eventual commercial-scale manufacturing. The ability to produce consistently high-quality MAPs at scale while adhering to regulatory standards is as important as the initial innovation itself. A forward-thinking approach to manufacturing is essential for derisking the technology and attracting investments needed to bring MAP products through late-stage clinical trials and to market.
The Science Behind Microneedle Array Patches
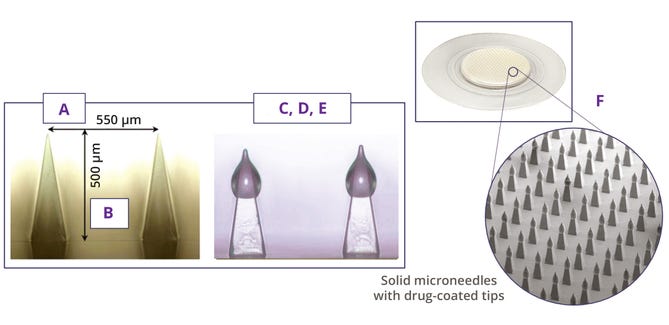
Each MAP consists of a backing layer that supports an array of microscopic needles designed to penetrate the stratum corneum, which is the outermost layer of human skin, to deliver drugs or vaccines to the epidermis or dermis. The needles’ small size reduces penetration depth and thus minimizes nerve stimulation, potentially resulting in painless drug administration — or at least causing less pain than traditional intravenous/intramuscular injections can cause (1).
Of the several types of microneedles, each has unique characteristics and applications. Solid-coated microneedles are coated with a drug formulation that dissolves upon insertion. Dissolving microneedles made from water-soluble materials dissolve completely to release a drug in a recipient’s skin. Hollow microneedles contain a hollow bore through which liquid drug formulations can be delivered. Hydrogel-forming microneedles swell upon insertion, creating channels for drug diffusion.
Intradermal delivery offers several advantages over traditional delivery methods for biologics. The dermis is rich in immune cells, making it an ideal target for vaccine delivery. In fact, a MAP approach could enhance vaccine efficacy (3). Additionally, rapid absorption of MAP-delivered drugs into a recipient’s bloodstream through the dermis enables faster onset of action than either subcutaneous or intramuscular injections can provide (4).
Another groundbreaking feature of MAP technology is its potential for room-temperature stability of products (2). That is made possible by solid-state formulations, which improve stability over that of liquid formulations because of lower moisture content. Use of stabilizing excipients (e.g., sugars) can help to preserve vaccine stability during drying and storage by immobilizing vaccine molecules. Fabrication of MAPs for vaccine delivery also can incorporate lyophilization steps to improve stability (5).
Room-temperature stability — or even a more modest reduction in required storage temperature — could alter fundamentally the logistics of transporting some MAP products, especially vaccines. By reducing or eliminating the need for cold-chain storage requirements, this innovation addresses one of the most pressing issues in worldwide healthcare: the difficulty of delivering vaccines to remote and under-served areas. The implications of such an advancement extend beyond just improving access; it also offers substantial environmental and economic advantages by decreasing carbon emissions and expenses linked to maintaining temperature-controlled supply chains.
Optimizing Microneedle Design for Larger Doses
Achieving consistent MAP delivery of large doses requires careful optimization of several key factors. Microneedle length, the number of needles per array, and array size and geometry all must be considered in optimizing MAPs to balance dose capacity and effective skin penetration. For instance, although longer needles would seem to be ideal for increasing dose capacity, they might not fully insert into skin due to its elasticity and related tissue compression.
Microneedle length plays a crucial role in determining the depth of penetration into recipients’ skin — and, consequently, the size of the drug payload carried (1). Longer needles can carry more volume, which must be balanced against patient comfort and the risk of reaching pain receptors or blood vessels.
Number of Microneedles Per Array: Increasing the number of microneedles on an array can make room for a larger total dosage. However, such an approach must be balanced against considerations of manufacturing complexity and the potential for a “bed-of-nails” effect in which force is distributed across too many points, reducing penetration efficacy.
Size and Geometry: The overall size of a microneedle array and the spacing between the needles can affect both the total dose capacity of the MAP and the consistency of drug delivery (6, 7). Larger arrays accommodate more and/or longer needles but may be less convenient for patients.
The shape and geometry of the microneedles themselves also plays a role in optimizing delivery. Sharper needles could penetrate more easily but offer less surface area for drug coating. Conversely, wider needles could carry more drug but encounter more resistance during insertion.
Ongoing research is focused on finding an optimal balance among all those factors to maximize dose delivery while ensuring consistent and effective penetration across diverse patient populations and application sites (2). As manufacturing capabilities advance, they increase the feasibility of complex and precisely engineered microneedle designs. New options include arrays with increased surface area and microneedle numbers, both allowing a greater total dose to be delivered. Further research on balancing surface area with geometry will open new possibilities for large dose delivery through MAPs.
Formulation Considerations for Solid-Coated Microneedles
The composition of coating formulations is crucial to ensuring effective drug delivery and stability for solid-coated microneedles. Typically, such formulations combine an active pharmaceutical ingredient (API) with excipients that enhance its solubility, improve coating quality, promote rapid dissolution upon skin insertion, and help to maintain stability during storage. Common solubility enhancers include surfactants such as polysorbates or sodium dodecyl sulfate (SDS), as well as cosolvents including ethanol, propylene glycol, and polyethylene glycol (PEG). MAP formulations also can include viscosity modifiers such as polyvinylpyrrolidone (PVP). Stabilizers include antioxidants, pH adjusters, and chelating agents. And other compounds such as sugars, sugar alcohols, and low–molecular-weight PEGs can be added to facilitate rapid dissolution (8). For example, one coating formulation that my company has worked with for solid-coated microneedles includes PVP (15% w/w) and sucrose (40% w/w).
The choice of excipients significantly influences both the stability and delivery efficiency of a coated drug. For instance, excipients can improve dissolution rates but in turn compromise long-term stability if they are not properly protected from moisture. Conversely, excipients that enhance stability could slow down API release from a microneedle coating. Here again, striking the right balance is essential for developing an effective MAP product (1).
Optimizing droplet size and position on microneedles is another critical aspect of formulation development. Larger droplets could be expected to deliver higher doses, but they can compromise a microneedle’s ability to penetrate skin effectively; smaller droplets positioned near microneedle tips have been associated with higher delivery efficiencies because they are more likely to be fully inserted into the dermis. Droplet position on a microneedle also can influence the rate of drug release and the depth at which a drug is delivered in skin.
Figure 1: Significant contributors to microneedle-array patch (MAP) drug-delivery efficiency include (a) space between needles, (b) length of needles, (c) droplet volume and/or solids per microstructure, (d) droplet shape, (e) formulation qualities, and (f) the number of needles per patch. An optimal design considers the interrelationships of these variables and how the right combination of these variables can result in a product that meets the target product profile.
Source: Peterson T. Manufacturing Microneedle Products with Regulatory Submission in Mind. Philadelphia Microneedle Conference, 7 September 2023; https://www.kindevadd.com/library/manufacturing-microneedle-products/?view_content=4702.
Precision Coating Techniques: Several coating methods have been developed for microneedle arrays, with dip coating and spray coating being among the most commonly applied. Dip coating involves precisely controlled immersion of microneedle arrays into a drug formulation, whereas spray coating uses fine atomization of a formulation onto a MAP surface. Each method offers advantages and drawbacks in terms of coating uniformity, reproducibility, and scalability. Controlling coating uniformity and reproducibility is crucial to ensuring consistent dose delivery across all microneedles in an array and between different arrays. That requires precise control of factors such as formulation viscosity, surface tension, and coating process parameters.
Scaling up coating processes for commercial production presents additional challenges. What works at laboratory scale may or may not transfer directly to large-scale manufacturing. Considerations here include maintaining coating consistency across larger batch sizes, reducing process variability, and ensuring that scaled-up processes meets regulatory expectations for uniformity, sterility, and purity.
Manufacturing Challenges and Solutions
Selection of materials for microneedle arrays is important, affecting not only the mechanical properties of the needles, but also their compatibility with drug formulations and skin tissues. Medical-grade polymers often are used for their strength, biocompatibility, and suitability to high-precision molding processes (9).
Injection molding is a preferred method for production of microneedle arrays because it can produce complex geometries with high repeatability (9). However, the process can bring difficulties during scale-up when precise dimensions must be maintained. Some costly, brittle materials used in laboratory-scale production are less than ideal for commercial scales (8). Addressing those considerations requires careful optimization of molding parameters: temperature, pressure, and cooling rates. Advanced mold designs incorporate features such as conformal cooling channels to help ensure uniform solidification and reduce cycle times (10). Keeping commercialization in mind from the onset of product development can enable selection of the most suitable materials and methods, reducing the need to adjust processes during scale-up.
Quality control (QC) is multifaceted in large-scale MAP manufacturing, involving both in-process controls and final-product testing. QC activities can include optical inspection for assessing microneedle geometry and coating uniformity, automated weight checks for dose verification, and rigorous testing of mechanical properties to ensure consistent skin penetration performance.
Interdependence among manufacturing steps adds an additional layer of complexity to MAP production. It is essential to balance each stage of manufacturing, with careful consideration for how each solution affects other decisions in later development phases. Maintaining such a balance requires taking a holistic view from the outset with a thorough understanding of the big picture.
Analytical Methods for Assessing Microneedle Performance: Coating uniformity typically is measured through a combination of techniques. High-performance liquid chromatography (HPLC) and enzyme-linked immunosorbent assays (ELISAs) provide quantitative analyses of drug content, scanning electron microscopy (SEM) enables visual inspection of coating morphology, and optical profilometry can measure coating thickness and distribution.
Correlating in vitro results with in vivo performance is an ongoing concern in MAP development. Although in vitro models can provide useful predictive data, factors such as skin elasticity, hydration, and biological variability will affect real-world performance. As more MAP products progress through clinical trials, the biopharmaceutical industry will gain valuable insights into such correlations, potentially leading to more predictive in vitro models.
Regulatory Considerations: The regulatory landscape for microneedle-based products is evolving as needed because these innovative delivery systems don’t fit neatly into existing categories. Regulatory agencies are working to develop specific guidance, and no MAP products yet have been approved by the FDA.
Sterility and bioburden control in manufacturing have been identified as critical considerations. Although terminal sterilization can be challenging for some drug-coated microneedles, implementing aseptic manufacturing processes and rigorous environmental controls will help to ensure product sterility. Active research and development (R&D) efforts are in progress toward novel sterilization methods that will be compatible with MAP products.
Scale-Up Strategies for Commercial Production: In addition to the technological and process considerations identified above, considering scale-up from the start will be crucial to MAP development success. Once data are made available to support the efficacy of this delivery method and its value in the market, it will be time to translate that potential into reality. Scaling from early stage development to GMP clinical manufacturing — and subsequently building to full commercial scale — is currently the main challenge to achieving the full potential of intradermal delivery.
Among contract development and manufacturing organizations (CDMOs), investment in manufacturing processes and approaches to enable investigational new drug (IND) applications is helping to bridge that gap. Linking clinical results to further development of new processes and technologies will enable manufacturing at increasing scales with necessary consistency. Such efforts will include creating flexible manufacturing platforms that can adapt to the unique requirements of microneedle production as well as exploring automation and solutions for sterile manufacturing.
In addition to technological knowledge and capabilities, companies must have the proper mindset when entering a MAP development program. Building on existing expertise will help them to face the technical challenges of the present moment while preparing for unexpected obstacles that arise in the future as microneedles achieve widespread adoption. Collaboration among technology developers, users, and researchers at every stage can streamline that process. That should include partnerships within the biopharmaceutical industry and with regulatory working groups to create and adapt evolving guidance documents.
On the Cusp of Adoption
Interest in intradermal delivery has increased steadily because of the numerous benefits it can offer to patients, clinicians, and the biopharmaceutical supply chain. Significant investment from governmental entities and nonprofit organizations underscores the growing recognition of microneedle technology’s potential, and that influx of funding has catalyzed substantial advancements in MAP technology through early to late stages of clinical product development.
For companies entering such a program, understanding how to scale manufacturing processes to meet GMP standards for both clinical and commercial production will be paramount. Addressing manufacturing challenges by increasing dose delivery, applying precision coatings, and implementing stringent QC will be essential to minimizing risk and attracting further investment needed to bring MAP products to market.
By focusing on manufacturing optimization, the industry can unlock the full potential of microneedle technology. That effort will involves not only refining current processes, but also innovating new methods to enhance production efficiency and product quality. The ultimate goal is to make MAPs a viable and scalable option for a breadth of therapeutic applications. That will enable the healthcare industry to realize fully the benefits of this technology and provide accessible treatments to patients around the world.
References
1 Avcil M, Çelik A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 12(11) 2021: 1321; https://doi.org/10.3390/mi12111321.
2 Kim Y-C, Park J-H, Prausnitz MR. Microneedles for Drug and Vaccine Delivery. Adv. Drug Deliv. Rev. 64(14) 2012: 1547–1568; https://doi.org/10.1016/j.addr.2012.04.005.
3 Ameri M, Lewis H, Nguyen J. Immunogenicity and Safety of Inactivated Influenza Split-Virion Vaccine Administered via a Transdermal Micro Needle System. J. Vaccines Immunol. 7(1) 2021: 41–48; https://doi.org/10.17352/jvi.000046.
4 Jung JH, Jin SG. Microneedle for Transdermal Drug Delivery: Current Trends and Fabrication. J. Pharm. Investig. 51(5) 2021: 503–517; https://doi.org/10.1007/s40005-021-00512-4.
5 Mistilis MJ, et al. Long-Term Stability of Influenza Vaccine in a Dissolving Microneedle Patch. Drug Deliv. Transl. Res. 7(2) 2017: 195–205; https://doi.org/10.1007/s13346-016-0282-2.
6 Waghule T, et al. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 109, 2019: 1249–1258; https://doi.org/10.1016/j.biopha.2018.10.078.
7 Loizidou EZ, et al. Evaluation of Geometrical Effects of Microneedles on Skin Penetration By CT Scan and Finite Element Analysis. Eur. J. Pharm. Biopharm. 107, 2016: 1–6; https://doi.org/10.1016/j.ejpb.2016.06.023.
8 Ingrole RSJ, Gill HS. Microneedle Coating Methods: A Review with a Perspective. J. Pharmacol. Exp. Ther. 370(3) 2019: 555–569; https://doi.org/10.1124/jpet.119.258707.
9 Luo X, Yang L, Cui Y. Microneedles: Materials, Fabrication, and Biomedical Applications. Biomed. Microdevices. 25(3) 2023: 20; https://doi.org/10.1007/s10544-023-00658-y.
10 Mizuno Y, et al. Fabrication of Novel-Shaped Microneedles To Overcome the Disadvantages of Solid Microneedles for the Transdermal Delivery of Insulin. Biomed. Microdevices. 23(3) 2021: 38; https://doi.org/10.1007/s10544-021-00576-x.
Andrew Riso is vice president of dermal delivery and licensing at Kindeva Drug Delivery (11200 Hudson Road, Woodbury, MN 55129; 1-800-643-8086), where he leverages over 10 years of expertise in strategic planning, financing, market research, and commercial analysis in biotechnology and drug delivery.
You May Also Like







