Development of a Single-Use Filling NeedleDevelopment of a Single-Use Filling Needle
Single-use components such as tubing, connectors, and filters have been widely used for many decades in bioprocess unit operations. Users have been able to identify and quantify the specific benefits of single-use over cleanable systems. In more recent years, many other process components have been designed for disposability such as bioreactors, mixers, and chromatography and ultrafiltration systems. Those and other advances have made it possible to incorporate multicomponent, presterilized manifolds into both existing and new processes, realizing benefits such as reduced cleaning, shortened set-up times, increased production throughput, and enhanced sterility assurance.
Although such developments have raised the possibility of developing fully disposable multistage processes, some unit operations have not been easy to convert to single-use because of limitations on scalability, costs, performance, and/or the unavailability of single-use options. One operation in which single-use technology is becoming more widely adopted is formulation and final filling. The industry is becoming more comfortable with single-use technology and gaining understanding of critical aspects such as extractables and leachables. In a 2012 survey of the bioprocessing market for single-use solutions, biopharmaceutical companies were asked to list those operations they considered to be suitable for such applications (1). Over half (54%) of respondents listed fill– finish operations.
One major challenge in developing disposable systems for final formulation and filling is replacing the stainless steel, cleanable filling needles with a cost-effective, single-use alternative. For systems incorporating stainless-steel filling needles, costs and risks associated with cleaning and resterilization of those needles must be evaluated, and those operations must be validated.
PRODUCT FOCUS: PARENTERALS
PROCESS FOCUS: FILL AND FINISH
WHO SHOULD READ: ANALYTICAL, MANUFACTURING, AND OPERATIONS
KEYWORDS: DISPOSABLES, VALIDATION, FACILITY DESIGN/ENGINEERING, LEACHABLES AND EXTRACTABLES, ASEPTIC PROCESSING
LEVEL: INTERMEDIATE
Single-Use Needle Design
Performance Requirements for Single- Use Filling Needles: When reviewing the properties and performance of current cleanable, stainless steel needles, Pall Life Sciences identified a number of critical requirements and attributes:
accurate and reproducible dose volumes
no drip formation, cavitation, or bubble formation during filling
accurate dosing for different surface tensions, densities, and viscosities
suitability for shear-sensitive formulations
low particulates with no preflushing
precise bottom-up, high-speed filling
a range of needle diameters for small and large volumes
compatibility with existing filling machines
tolerance for sterilization by steam autoclaving ≤130 °C and gamma irradiation ≤50 kGy
robust and rigid construction
suitability for use according to pharmaceutical good manufacturing practice (GMP) regulations
compliance with appropriate pharmacopoeial tests.
Taking into account those performance attributes along with other considerations, the company defined two objectives in this product development project. First, it needed to meet the basic performance requirements for a disposable filling needle. Second, it needed to incorporate unique features such as suitability for products that are sensitive to stainless steel and improved accuracy and more consistency of fill volumes compared with cleanable needles.
Design Features: The filling-needle design was based on five primary features:
organic polymers for all fluid-contact surfaces
suitability for steam autoclave and gamma-irradiation sterilization
filling accuracy equal to or better than other available filling needles
validatability for pharmaceutical dosage filling
“plug-and-play” usability without precleaning or preconditioning.
Figure 1 shows the resulting product design from that development program, and Table 1 lists key features, materials of construction, and properties of the filling-needle design. This design and construction provides good needle rigidity while strictly controlling shaft straightness, lumen diameter, wall thickness, and surface smoothness. All materials were selected to comply with pharmaceutical GMP regulations and standards. Polymeric fluid contact surfaces also ensure suitability for filling of products that are sensitive to stainless steel. The Luer fitment design also enables secure fitting of these needles into a range of existing filling machines and their incorporation into presterilized, single-use manifolds and systems.
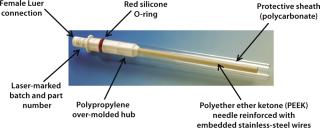
Figure 1:
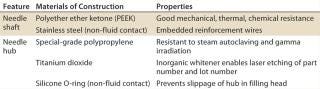
Table 1:
Performance Qualification and Validation
The company performed a series of comprehensive qualification studies as part of product validation for the filling needles. It is not possible to describe these studies in detail herein, but some critical tests and results are highlighted below. You can find more information in the product validation guide available from Pall (2).
Validation Program: A validation program was designed to demonstrate the mechanical and functional performance of these single-use needles as well as evaluate their levels of extractables, fluid-path cleanliness for particulates and endotoxins, and biological safety. Summarized schematically in Figure 2, the program covered desired features of the product and the possibility of using presterilized needles directly in a filling system (unlike reusable stainless-steel needles).
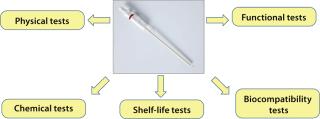
Figure 2:
Physical Tests: Leak testing verifies the integrity of the joint between a needle’s shaft and its overmolded hub. For this determination, each unit was closed at one end and pressurized with air at 1,100 mbar so the pressure decay could be recorded. The company defined a maximum allowable leak rate as that corresponding to the absence of any visible leak when a unit is placed in a water bath. All samples passed this test, confirming that the joint’s integrity is not compromised by autoclaving or gamma irradiation up to the maximum allowed levels (130 ± 2 °C for 75 min and 50 ± 5 kGy, respectively).
Dimensional accuracy tests verify that a needle’s collar diameter and Luer-lock connection with the verticality of its shaft position relative to the Luer-lock connection are all within acceptance specifications after autoclaving or gamma irradiation. All samples passed these tests, confirming dimensional accuracy and stability of the needles after sterilization.
A burst-pressure test was designed to assess the strength and mechanical properties of needles after different sterilization pretreatments. All burst gauge pressures were >35.6 bar (517 psig), confirming a very high safety factor of at least 35× over the recommended operating gauge pressure of 1 bar (14.5 psig) even after pretreatment by sterilization and storage at elevated temperatures.
Functional Testing: A volumetric accuracy test was designed to measure the accuracy of minimum and maximum fill volumes after different pretreatments of the needles and also after 24 hours use. Figure 3 shows the test rig used here. It contains a water reservoir within a temperature-controlled water bath. A filling machine delivered water to a needle that was positioned above precision weigh scales for volume measurement by weight. Ten dispensed volumes were measured for each test needle at its minimum and maximum volume settings. Results show that all needles were within the stated specification of accuracy at both the lower and higher limits of the target volumes (Table 2). Similar results were also obtained after 24 hours of use.
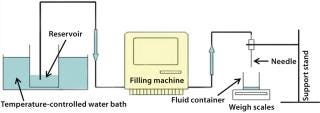
Figure 3:
Table 2:
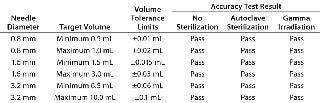
Table 2:
Chemical and Biocompatibility Tests: The company’s approach to validation of single-use components and systems for extractables, cleanliness, and biological safety are presented in detail elsewhere (3,4,5). Similar validation studies were performed on these Allegro single-use needles to ensure that they would be suitable for pharmaceutical use — and to provide data for users to include in their submissions for regulatory approval.
I can only summarize the validation approach and results here. Find more detailed information in the Allegro single-use needle validation guide (2).
The Bio-Process Systems Alliance (BPSA) has published useful recommendations on testing for extractables from single-use systems (6, 7). Based on those recommendations, Pall developed and applied a comprehensive program for evaluation of extractables from these needles and their potential impact on leachables in final products (3,4,5). The program provides generic qualitative and quantitative extractables data on single-use products and was used for determination of “generic” extractables of Allegro single-use needles.
For process-specific extractables and leachables studies, the company used a risk-based approach incorporating its model solvent approach (8). Generic extractables on Allegro needles were obtained using water and ethanol extracts, which were analyzed for a range of organic and inorganic compounds using 12 different analytical methods (Table 3). See the product validation guide for full details of the analytical results (2). Testing found no genotoxic compounds from autoclaved or gamma-irradiated needles. The levels of extractable compounds detected were extremely low (in many cases at or near the limits of detection).
Table 3:
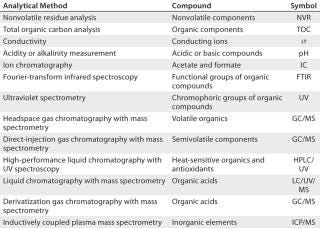
Table 3:
Extractables levels were tested after repeated extractions. Filling needles typically are positioned at the final stage of a process in which leachables could potentially have an impact on product purity, especially after contact with process fluids for the entire filling time. To assess the stability of potential leachable levels over time, the company performed a further aqueous extractables study on autoclaved or gamma-irradiated needles with 10 consecutive water extractions. Total organic carbon (TOC) analysis of the extracts served as an indicator of extractable levels. Results show (Figure 4) that TOC levels remained constant throughout the study, confirming that extractables remain low and stable over time. These results (along with other data on cleanliness and biological safety described below) support the use of these needles without preflushing before use.
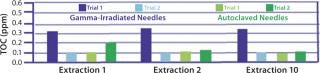
Figure 4:
Pall subjected Allegro needles to its standard range of tests for validation of other single-use products to confirm product suitability for pharmaceutical processes. Table 4 summarizes this test program and the results obtained. See the validation guide for full details (2)
Table 4:
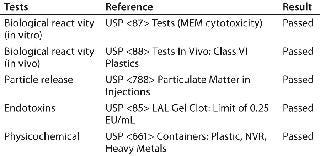
Table 4:
Application Results
My company performed further studies on Allegro single-use needles. Disposable-Lab SAS is a contract manufacturer based in Bordeaux, France, that develops innovative processes for producing sterile pharmaceutical products. Its aseptic filling operations and expertise offered an ideal site for application studies using Pall‘s single-use needles. Performed on a filling line with an automated time/pressure dosing system, this trial had the following objectives:
to measure fill-volume accuracy with water for injection (WFI) using different-size needles and a range of fill volumes
to perform comparative studies against commercially available stainless-steel, cleanable needles
to investigate fill-volume accuracy of single-use needles with both viscous fluids and liquids with low surface tension.
Fill-Volume Accuracy Trials with WFI: This trial evaluated fill volumes in the range of 0.5–10 mL using WFI. Figure 5 shows results from a small-volume fill using a 1.6-mm needle for a target volume of 0.68 mL. The system gave a consistent fill volume over a run of 53 fills. A large-volume fill with a 3.2-mm needle and a target volume of 8.45 mL gave similar results (Figure 6).
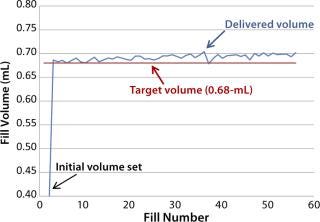
Figure 5:
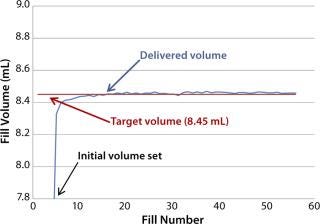
Figure 6:
Table 5 presents additional data from the volume-accuracy trials, showing mean dispensing times at constant pressure for each needle diameter and target fill-volume combination. Low standard deviations and variation coefficients confirm a high and consistent accuracy throughout the range of target volumes tested.
>Table 5: /p>
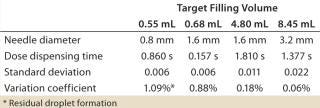
Table 5:
Comparative Trials: Having demonstrated accurate and consistent fill volumes with the Allegro single-use needles, Disposable-Lab believed it was important to establish how their performance compared with commercially available stainless-steel needles. Using a similar test set-up to that for the accuracy trials (with WFI as the test liquid) the laboratory compared Allegro single-use needle with two types of stainless-steel needles using needle diameters recommended for the specified fill volumes. Figure 7 shows the results of this trial. Summarized in Table 6, these results show that the Allegro needle gave a more accurate and more consistent fill volume, as evident from its lower standard deviation. No drip formation was observed with the Allegro needle, but drips were present with stainless-steel needle B.
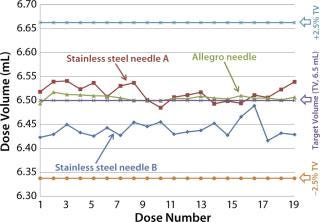
Figure 7:
Table 6: /p>

Table 6:
Trials with Different Fluids: Having established the accuracy and reproducibility of single-use needles with WFI, Disposable-Lab evaluated the single-use needles’ performance with liquids of different viscosity and surface tension. This involved the following test liquids:
a viscous, aqueous solution (3–4 cP), such as that typically associated with ophthalmic products, injectable suspensions, and blood products.
a solution with low surface tension (25.2 mN/m) typically associated with formulations containing surface-active agents (e.g., emulsified products) or organic solvents (e.g., ethanolic formulations).
Under identical test conditions to those used above, the results show that these needles gave consistent and accurate fill volumes for both the viscous and low–surface-tension solutions (Table 7). The needles gave similar results when tested on other types of filling equipment.
Table 7:

Table 7:
Implementation Case Study
Disposable-Lab SAS has recognized over the past 10 years that an increasing number of injectable biotechnology products are in development. The company also recognizes that single-use components and systems are ideally suited for processing such products. This presents the possibility of creating — from the ground up — the first-ever production facility using entirely disposable process systems for formulation and filling operations.
The company has thus embarked on a development program to create a new manufacturing facility exclusively dedicated to single-use processes. This modular “plastic factory” is designed for production of sterile products both for clinical trials and small commercial batches. Disposable-Lab SAS selected two principal partners to assist in this development: a company specializing in single-use isolators; and Pall Life Sciences for single-use needles and for air and product filtration technologies.
Design of Aseptic Single-Use Filling Line: Figure 8 schematically represents the aseptic filling line. The development program focused on eliminating risks from product cross-contamination, microbial contamination, and particulate contamination.
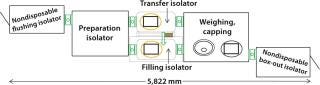
Figure 8:
Product Cross-Contamination: In a multiproduct manufacturing plant using a traditional cleanable filling system, the risk of product cross-contamination must be mitigated through strict control of cleaning, system sterilization, and other procedures. Development of a fully disposable, presterilized filling system has enabled my company to eliminate such procedures and provide a high level of assurance of product purity and sterility as well as savings in time and cost.
Isolators: The Disposable-Lab installation was designed to ensure that, wherever possible, aseptic filling unit operations could be performed in a closed microenvironment using disposable isolators. The company’s specially designed isolators have single-use, flexible plastic walls and gloves and are linked by transfer interfaces (Photos 1). The only nondisposable item in the product transfer pathway is the flushing-isolator box — and optionally, the box-out isolator box.
Photos 1:
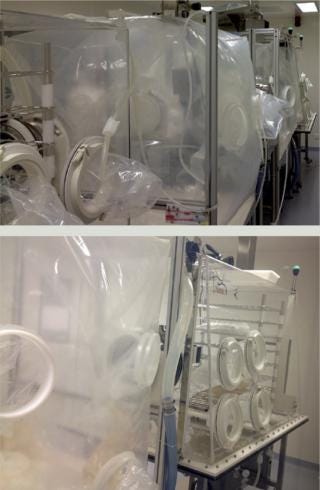
Photos 1:
Filling Process: The enclosed filling chamber (Photo 2) contains an Allegro single-use filling needle and filling head for constant pressure and timed delivery of sterile product. Sensors provide continuous communication and control of the process within this filling chamber. A mobile support table for vials baskets enables their transfer into a weighing and capping chamber. Unlike other filling systems, no mechanical constraints are present, making this system suitable for fragile and shear-sensitive products.
Photo 2:
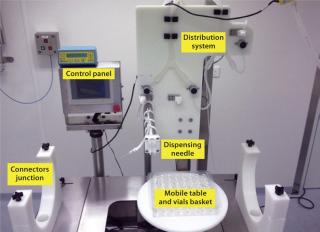
Photo 2:
Microbial and Particulate Contamination: All isolators are presterilized by gamma irradiation, including the gloves in the flushing isolator and the exit chamber.
To meet regulatory requirements, aseptic filling demands strict control over the size and quantity of particulates in filled sterile products. In conventional reusable filling systems, filtered laminar air flow within the filling zone is essential to remove airborne particles generated by the system. Disposable- Lab SAS has developed the concept of isolators without laminar flow. They are fabricated under ISO 5 conditions with strict manufacturing controls. In addition, the disposable system has been designed to generate no particles during a filling process. To confirm the particle cleanliness of this system, the company evaluated particulate levels in the filling zone using an online air-particle monitor for the entire duration of a filling process. All samples showed levels below the maximum permitted for aseptic filling. In addition, the company performed stability studies. Filled product after five weeks’ storage at 56 °C for five weeks was analyzed for particle levels. Table 8 lists data from a typical certificate of analysis, confirming that those levels are within specified limits. No out-of-specification (OOS) results were obtained for any sample tested.
Table 8:
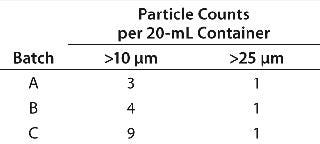
Table 8:
Manufacturing Environment and Services: The single-use filling line operates in a class C (ISO 3) cleanroom environment supported by the following utilities and services:
Steam sterilizer and dry heat oven (Fedegari Autoclavi SpA)
Washing machine (Steelco SpA) — exit protected by laminar air flow
Distillation plant for WFI (Stilmas SpA)
WFI circuit (Sterigene).
Case Study Summary: My company’s concept of developing a new facility incorporating fully disposable filling systems for sterile injectables has been realized. The plant is now fully operational for fill and finish of injectable products (Photo 3). Process qualification and validation have shown that single-use technology has effectively eliminated the risks of cross-contamination, microbial contamination, and particulate contamination. Other benefits of this approach now can be realized: faster set-up and turn-around times; increased production flexibility; and lower expenditure on utilities such as steam, chemicals, and WFI.
Photo 3:

Photo 3:
Discussion
The benefits of converting from stainless-steel to single-use filling needles will vary by process and facility. For a start-up operation such as that described above, single-use may be a very cost-effective option. Similarly, for contract manufacturing organizations (CMOs) that handle many different product formulations, elimination of cleaning and facilitation of change-over might favor single use. But time savings play an important role for everyone considering single-use options. The greatest savings in time come when single-use needles form part of a single-use filling manifold (Figure 9). That eliminates time taken not only for cleaning but also for set-up and disassembly.
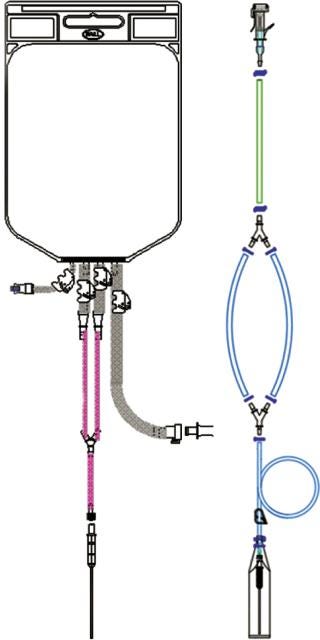
Figure 9:
The schematic process flow sequence in Figure 10 gives a broad indication of comparative process times for a cleanable system and a single-use manifold system. When converting those timings into costs, other cost factors also should be considered: e.g., WFI generation, disposal of consumables, labor and analytical costs, and cleaning validation. Other considerations — such as sterility assurance levels and contamination risks — are more difficult to quantify but can affect the decision process in favor of a single-use approach.
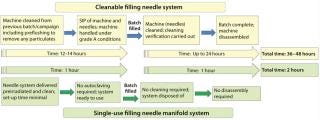
Figure 10:
A Model System
Here I have described the approach to design, development, and qualification of a unique single-use needle for final filling of liquid formulations. It was evident early in the project that making a needle that offers significant benefits over reusable stainless-steel needles would require an innovative design and good engineering practice (9). Validation studies formed an essential part of the development program to ensure that the new technology would meet regulatory requirements and perform to specifications accurately, consistently, and reliably.
The opportunity to perform beta trials in a fully operational production facility was a key component of that program. The Disposable-Lab trials confirmed that accurate and consistent fill volumes could be achieved for a range of set volumes using different process fluids in a production environment. In addition, comparisons against stainless-steel needles demonstrated that the single-use needles offered greater accuracy and consistency of fill volume.
The case study presented herein shows how a fully disposable aseptic filling system operating in a purpose-designed factory dedicated to single-use processing can offer a practical alternative to conventional, cleanable systems. The single-use system eliminates risks of product cross-contamination, microbial contamination, and particulate contamination — and helps the company realize other benefits for its manufacturing processes.
Finally, potential savings in process time compared with a cleanable system have been calculated for a model system. The basis of these calculations should help other users calculate the potential savings and perform cost/benefit analyses for their own specific processes.
Author Details
Jean-Pascal Zambaux is president of Disposable-Lab SAS, 7 Allée Isaac Newton, 33650 Martillac, France; 33-557-12-0732; [email protected]; www.disposable-lab.com. James Barry is marketing manager for Allegro single-use systems at Pall Life Sciences, Harbourgate Business Park, Portsmouth, UK; 44-2392- 338184; [email protected]; www.pall.com.
REFERENCES
1.) 2012. Third Annual Survey of the Bioprocessing Market for Single Use Solutions, Aspen Brook Consulting, LLC, Park City.
2.) USTR 2387 2013.Validation Guide: Pall Allegro Single-Use Filling Needles, Pall Life Sciences, East Hills.
3.) Ding, W, and J Martin. 2008. Implementing Single-Use Technology in Biopharmaceutical Manufacturing: An Approach to Extractables/ Leachables Studies, Part One — Connectors and Filters. BioProcess Int. 6:34-42.
4.) Ding, W, and J Martin. 2009. Implementation of Single-Use Technology in Biopharmaceutical Manufacturing: An Approach to Extractables/ Leachables Studies, Part Two — Tubing and Biocontainers. BioProcess Int. 7:46-51.
5.) Ding, W, and J Martin. 2010. Technology in Biopharmaceutical Manufacturing: An Approach to Extractables and Leachables Studies, Part Three — Single- Use Systems. BioProcess Int. 8:52-61.
6.) 2007. Extractables and Leachables Subcommittee of the Bio-Process Systems Alliance BPSA. Recommendations for Extractables and Leachables Testing, Part 1: Introduction, Regulatory Issues and Risk Assessment. BioProcess Int. 5:36-44.
7.) 2008. Extractables and Leachables Subcommittee of the Bio-Process Systems Alliance BPSA. Recommendations for Extractables and Leachables Testing, Part 2: Executing a Program. BioProcess Int. 6:44-52.
8.) Ding, W, and J Martin. 2008. Single-Use Technology: An Approach to Extractables/ Leachables Studies — Connectors and Filters. BioPharm Int. www.pall.com/pdfs/Biopharmaceuticals/bp1208_PallAppNote.pdf:14-15.
9.) Botterill, M, and B Rawlings. 2008. Applying Good Engineering Practice to the Design of Single-Use Systems. BioProcess Int. 6:18-25.
You May Also Like






