Large-Scale Freezing of Biologics October 2009Large-Scale Freezing of Biologics October 2009
Production of biologics is an expensive process, and to optimize capacity use, bulk protein solution is often produced in manufacturing campaigns. It is converted into drug product based on market demand and therefore may have to be stored for relatively long periods. To decouple the bulk solution production from that of the final drug product, bulk is often stored frozen.
Transport of frozen bulk product between sites offers several practical advantages over its transport in the liquid state (2–8 °C). Maintaining 2–8 °C requires accurate control systems to ensure that a product does not get too cold and (partially) freeze. A liquid shipment also subjects protein to greater degrees of agitation stress at air–liquid interfaces. So a successful bulk storage program will enhance bioprocess capacity use and reduce overall cost of production. However, success requires careful consideration of biophysical and engineering principles in development of a frozen-storage operation and its impact on the product to be frozen.

Figure 1:
PRODUCT FOCUS: PROTEINS
PROCESS FOCUS: FORMULATIONS AND DOWNSTREAM PROCESSING
WHO SHOULD READ: PROCESS/PRODUCT DEVELOPMENT, FORMULATORS
KEYWORDS: STABILITY, AGGREGATION, BULK INTERMEDIATES, EUTECTIC, STORAGE, TG, TRANSPORT, DENATURATION
LEVEL: INTERMEDIATE
Publicly available information suggests that nearly half of commercial biotherapeutics are stored frozen (1). Given the high value of product being processed in the freezing operation, it is surprising that there is little scientific guidance available for practitioners. Literature on the impact of protein freezing is limited to very small-scale experiments that, although useful, do not address complications created by the relatively large heat and mass transfer dimensions of practical large-scale systems. The subject is expensive for academic research and not interesting enough from a results perspective because real-time, long-term stability studies are required. Unlike liquid-state stability studies, these cannot be meaningfully accelerated. A lot of knowledge resides in the industry, and freezing practices are often empirical.
This two-part review discusses the basics of freezing biologics relevant to large-scale processes (part one) and critically examines some technologies and systems available to provide guidance on rational development of this unit operation (part two). An abridged version of these two articles has been published previously (2). For our purposes, we are limiting consideration of biologics to protein biotherapeutics only.
Fundamental Aspects of Freezing
Proteins can undergo degradation by many mechanisms. However, the primary mechanism of concern with frozen storage is aggregation, although some chemical-reaction–based mechanisms could arise with certain susceptible proteins. Knowledge of the fundamentals of freezing and thawing is geared toward understanding their impact on and the prevention of (permanent) structure loss and concomitant formation and growth of aggregates.
A discussion of the low-temperature behavior of proteins must distinguish between the effects of the low temperature or cold per se from that of the freezing process itself. Franks distinguishes the former as “impact of chill,” which involves biological changes produced by changes in the property of liquid water with temperature (e.g., ionization constant, dielectric constant, hydrogen bond energies, and hydrophobic interactions) leading to hydration of the hydrophobic core (3,4). Generally reversible protein structure changes that occur as a consequence of low temperature are termed cold denaturation.
The freezing process, however, subjects proteins to other stresses as a consequence of the removal of water as ice. The resulting cryoconcentration and desiccation of protein can be classified as osmotic stresses. Other freezing-process–induced stresses include ice interface formation, pH changes, and phase separation. Protein structure changes that occur as a consequence of such stresses have a greater probability of being irreversible, and are classified as freeze denaturation (3,4).
Rate of Cooling: Freeze–thaw behavior of proteins has been studied extensively but primarily in microscopic or small volumes (a few milliliters or less), often in conjunction with lyophilization. Among the parameters considered important are the rate of processing (cooling and heating) and composition. Effects as presented in the literature provide no clear guidance because the terms fast and slow are specific to each study, and rates (if) reported vary widely (5). Further, the use of minimal volumes makes the “process” aspect of literature studies difficult to extrapolate to freezing large-volume bulk protein solutions.
The “rate” of cooling in practical systems comes into play indirectly through its effect on the nature of the ice interface created. Moreover, rate as often used in the literature refers to the drop in temperature over time. But the more important rate relates to the time required for a solution to actually freeze and transition from liquid to a solid or glass phase (indicated as freezing time or duration in Figure 1). Any practical system larger than a few hundred milliliters will freeze over a period as the process progresses, thus producing much more complex behavior than can be described by a single rate parameter. Although beyond our scope here, it is clear that geometry (heat and mass transfer dimensions) is important to the outcome of the process as well.
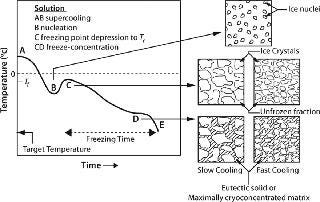
Figure 1: ()
Osmotic Stresses — Dessication and Cryoconcentration: As an aqueous solution freezes, conversion of water into ice causes progressive freeze-concentration (cryoconcentration) of the unfrozen mixture of solutes (including the protein) because growing ice crystals will exclude solutes. The increasing concentration of solutes in the unfrozen fraction results in a continuously decreasing freezing point (Figure 1, CD). Progressive freeze-concentration of the unfrozen mixture leads to an increase in viscosity, reduction in diffusion coefficients, and ultimately a glassy matrix when no more water can crystallize. If all the freezable water is converted to ice, then the concentration of solutes can become very high.
In the case of crystallizable excipients (e.g., NaCl), reaching a solubility limit during cyoconcentration could cause them to crystallize out of solution, forming a eutectic. For a 150-mM (~0.9% w/w) NaCl solution, the eutectic at −21.2 °C has a concentration of 23.3% w/w, a ~25-fold increase (Figure 2) (6). Most carbohydrates (e.g., sucrose) tend to be noncrystallizable, so their maximal freeze-concentrated (equilibrium) concentration is around 80% w/w (Figure 3) (7,8). General trends in the cryoconcentration behavior of easily crystallizable excipients can be followed using an equilibrium phase diagram (if available). For noncrystallizable excipients, cryoconcentration behavior is better described by state diagrams instead because practical freezing processes are seldom carried out under equilibrium conditions. Combinations of crystallizable and noncrystallizable excipients will give intermediate behavior depending on the ratio of the two (9). Figure 1 shows a freezing curve with schematics showing the nature of frozen materials in solution at various studies.
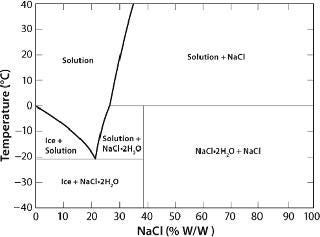
Figure 2: ()
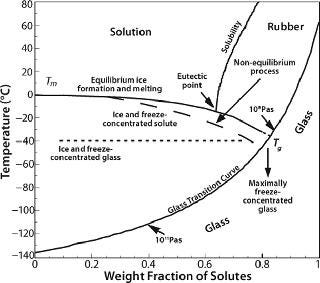
Figure 3: ()
In practical systems, a small amount of supercooling is followed by rapid ice nucleation throughout the bulk solution, so not all the solutes are pushed toward the geometrical center. However, because of the heat transfer distances involved, ice growth is faster at the walls than in the center, so some diffusion of solutes toward later-freezing regions occurs. Excluded solute at the interface depresses the freezing point of solution just in front of it to below the freezing point of the remaining nominal bulk solution, creating a situation in which solution further from the ice front is in a supercooled state (constitutional supercooling). This leads to instability in the ice front, increasing the probability of dendritic ice formation because protuberances can better shed latent heat into larger and supercooled volumes of liquid (10).
Dendritic growth of ice crystals enables entrapment of solute in unfrozen channels between the dendrites. However, solutes trapped between those ice crystals still experience a localized cryoconcentration effect as water is extracted and the channel narrows by growth of ice. Thus, concentration gradients are generated and “frozen-in.” Figure 4A shows an example of concentration gradients in a bottle containing a monoclonal antibody (MAb) solution. Such gradients can persevere if thawing is carried out without mixing (Figure 4B).
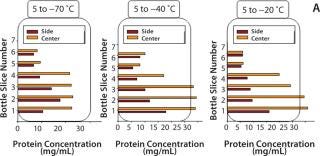
Figure 4a: ()
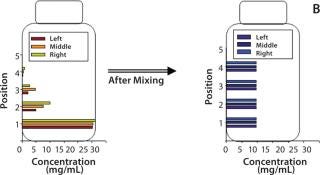
Figure 4b: ()
Our in-house observations show that proteins and other excipients cryoconcentrate to nearly the same extent: The impact of differences in diffusion coefficients is overridden by convective effects. Convective effects also become important in large-scale systems, where temperature differences create density gradients in the unfrozen solution. The excluded solute just ahead of the ice front has a high concentration of solutes (and thus a higher density) than the bulk. Density gradients create a convective flow that carries solutes down toward the bottom of the container. The overall effect depends on the container’s depth and geometry.
The flip side of cryoconcentration is desiccation. As freezing proceeds, removal of water (as ice) dries out of the protein in the amorphous (glassy) phase. Each protein molecule structures the water molecules around it, with a first hydration shell of water molecules (~0.3 g/g) directly attached to the protein. The water molecules aid in proper folding and lubricate movement of a protein’s amino-acid backbone and side groups by rapid formation and exchange of hydrogen bonds (11). Hydration is thus important for maintaining the three-dimensional structure of a protein.
The entire hydration shell of the protein is unlikely to be completely removed by freezing: Part of it is unfreezable or bound water, and outer shells or loosely bound water can be lost. Structural changes have been recorded for lysozyme (amide I band–narrowing implying protein–protein interaction) as water is removed during freezing in the absence of cryoprotectants such as sucrose (12). The steepest changes were recorded at low hydration levels under ~15%. Structural change was prevented with 10% sucrose, which satisfied the protein’s hydrogen bonding requirements.
Cryoconcentration Consequences: Ice formation and consequent cryoconcentration affect a frozen biologic in a number of ways. Increasing ionic strength during freezing can reduce its solubility and potentially also destabilize protein structure through disruption of salt-bridges. Buffer salts can crystallize if their concentration limit is reached, which changes pH. Among common buffers used for biologics, the sodium phosphate buffer mixture is particularly susceptible: pH can change from 7 to ~4 on precipitation of the dibasic salt below 0 °C. Even if the salts do not reach their solubility limits, their pKa value is sensitive to temperature, so pH shifts will occur during freezing and in the frozen state. Data are available for pKa/temperature in the liquid state (13). In general, dissociation constants for carboxylic and inorganic acids have dpKa/dT values close to zero. They are slightly negative (~0.01) for secondary amines and more negative (~0.015–0.02) for primary amines. Changes in pH in the frozen state are not predictable, but some data are available (14,15).
Other excipients (e.g., mannitol, glycine, and polyethylene glycol) in a formulation can crystallize and/or phase separate during freezing. As in all situations involving equilibrium in practical processes, the actual extent of crystallization of buffer or other solution components depends on volume, cooling rate, the presence of other solutes, initial concentrations, and nucleation rates. Recently, liquid– liquid phase separation of a protein-rich from a protein-poor phase has been reported in a high-concentration MAb solutions in high ionic strength buffer when cooled to 0 °C (16,17). A number of proteins display this effect (18,19,20). High-salt and/or high-sequence hydrophobicity (21) contributes to the accessibility of this transition. Cryoconcentration could trigger this in susceptible protein systems with low salt content as well.
Cryoconcentration can also affect reaction rates. Reduction in temperature lowers the rate of degradation reactions (the Arrhenius effect), but cryoconcentration can counteract that through an increase in the concentration of reactants. So reactions such as oxidation can be enhanced, especially when the solubility of oxygen increases as temperature drops while ice formation also excludes gases. Dissolved oxygen at high concentration can be trapped along with proteins in the final glassy matrix. Other potential incompatibilities among solutes and impurities (e.g., peroxides, trace metals) could also be exacerbated.
At the Ice Interface: Proteins also interact with the ice surface, resulting in perturbation of their native structure. In effect, they can (partially) denature at the ice interface through weakening of their hydrophobic bonds as well as adsorption onto the ice surface (22,23). The extent of structural perturbation depends on the rate of cooling or heat removal, which determines the number of nuclei, the size of ice crystals formed, and the interfacial area generated (Figure 1).
Protein–ice interaction lowers the free energy of the denatured state more than that of the native state. Interactions are therefore stronger for an expanded protein with a larger solvent-accessible surface area, which aids in adsorption (24). A strong positive correlation has been shown between freeze denaturation and surface denaturation (23). When freeze–thaw cycling studies are performed to identify cryoprotectants (including surfactants), the primary mechanisms stabilized against are surface-induced denaturation and cold denaturation.
Cold Denaturation: As the temperature of a solution drops, properties of the aqueous solvent medium change including its dielectric constant, acid/base ionization constants, diffusion rates and mobility, solubility of hydrophobic residues, and hydrogen bond energies. Those changes in themselves, without the complicating effects of freezing and phase changes, cause reversible changes in protein structure and cold denaturation. This low-temperature effect (“chill”) is distinct from the effect on protein structure that comes from the actual freezing (e.g., cryoconcentration, phase changes, and ice surface denaturation) discussed above and elsewhere (3,25,26).
A more precise thermodynamic explanation for cold denaturation comes from considering the free energy of protein unfolding. Cold–induced unfolding (cold denaturation) is a physical consequence of the temperature sensitivity of noncovalent electrostatic and hydrophobic interactions, which become weaker at lower temperatures (3,27,28). It is a thermodynamic consequence of the large and positive ΔCp of proteins unfolding (and which, within experimental error, can be considered a constant for each protein) (29). Cold-denaturation temperature has never been experimentally observed because it generally lies below 0 °C, at which point the chill-induced effect is difficult to separate from that of freezing itself (30). However, changes in solvent conditions such as pH, addition of chaotropic agents, and other perturbants have been used to make the cold-denaturation temperature more accessible (31,32,33,34). From a pharmaceutical perspective, commonly added sugars and polyols move the cold-denaturation temperature lower, thus stabilizing a protein molecule (35).
In practical terms, the reversible nature of unfolding due to chill-induced cold denaturation is not in itself detrimental to the storage stability of a protein. If the stress were strictly limited to cold denaturation, then stability in the frozen state probably would not be an issue. However, chill-induced unfolding probably makes a molecule more susceptible to freeze-induced stresses, leading to aggregate formation and/or loss of structure.
Storage, Shipping, and the Glass Transition Temperature: Once formulated and frozen, a protein must be stored and remain stable over long periods — extending into years. When freezing is complete, the protein and other solutes are concentrated into a highly viscous amorphous matrix with a characteristic temperature called the glass transition temperature (Tg), above which the matrix is regarded as “rubbery” and below which it is “glass” (Figure 3). The actual transitions are not so distinct, but molecular relaxation and related viscosity and mobility phenomena show a Williams–Landel–Ferry (WLF) type of dependence just above the Tg, which has a much greater sensitivity to temperature than in an Arrhenius relation (36). Viscosity decreases dramatically in the vicinity of and above Tg. Thus, even if the matrix appears nominally frozen in this temperature region, diffusive processes can occur over long time-scales.
The storage temperature for a frozen matrix must be set based on Tg with some margin because even reduced molecular mobility can affect the stability of protein over long time scales. The US Pharmacopeia defines the −20 °C condition as a −10 to −20 °C range, whereas the Ph. Eur. defines it as −20 ± 5 °C. Periodic fluctuations on the high side of the storage temperature can speed up diffusive processes, and large deviations will negate the effect of a low storage temperature. Storage temperature must be chosen such that the high-temperature part of the cycle and the time spent above the set-point will still provide an acceptable product. If data are available, then mean kinetic temperature estimations can be applied to determine the acceptable range.
In a well-designed formulation, freeze-induced structural perturbations will be largely reversible upon thawing, although some fraction may become irreversibly damaged. More important, depending on the storage temperature in relation to Tg, some minor loss of structure in the frozen state may cause aggregate formation over time when (partially) unfolded molecules serve as nuclei and interact with their neighbors if mobility is sufficient. Thus, storage near and above the Tg of the matrix will allow denaturation and/or aggregation to progress, albeit slowly. Ice crystal size and morphology can also change if mobility is sufficient. That leads to crystal growth through Ostwald ripening and to a redistribution of solutes. Crystallizable excipients (e.g., NaCl, mannitol, glycine) trapped in a nonequilibrium state during freezing can crystallize over time given the mobility allowed by storage above Tg. Phase transitions over time have been shown in the frozen state, recently reported for sorbitol, leading to protein aggregation as the cryoprotective effect of excipients is lost because of crystallization (37).
The type of container can potentially also affect frozen-state behavior through adsorption, depending on the hydrophobicity of its interior surface, although other factors could be confounding the reported results (38). However, most practical systems will have low surface areas in relation to their volume, which reduces the relative impact of the container interface compared with other factors discussed here. Similar to storage temperature, a shipping temperature must be chosen with regard to the properties of the glassy matrix. Shipping below Tg is desirable.
Formulation Composition and Tg: It is clear that Tg is a key bulk formulation parameter. The ability to manipulate it can thus be useful in enhancing the stability of a protein in a frozen matrix. A solution’s Tg depends on the nature of the glass formers involved and their concentrations in the glass. The main components of protein formulations are buffer salts, stabilizers and/or cryoprotectants, and the protein itself. Assuming the additivity of their properties (free volume), Tg is given by the Gordon–Taylor equation or its simplified version, the Fox equation:
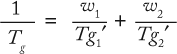
where Tg is the glass transition temperature of the solution, and wi and Tg′i are the weight fractions and glass transition temperatures of the solution components.
Commonly used stabilizers in protein formulations include sugars, polyhydric alcohols, certain amino acids, and higher oligosaccharides. Their utility in the frozen state comes from their inability to crystallize and a lack of eutectic phase separation. Most such excipients are subject to vitrification or glass formation as freezing proceeds, crystallizing excipients being generally unsuitable. A comprehensive compilation of Tg is available (39,40) although some values are disputed (41). Tg values generally increase with molecular weight (7,8). Proteins in solution do not crystallize on cooling. Being polymeric, they also exhibit a glass transition temperature, which tends to range around −10 °C regardless of protein size and structure: −11 °C for ovalbumin, −13 °C for lysozyme, −15 °C for myoglobin, −11 °C for BSA, and −9 °C for lactic dehydrogenase (42). The Tg for pure water is generally accepted to be ~135–140 K (about −135 °C), at the lower end of the glass-transition curve (Figure 3).
Knowledge about Tg provides a strategy to adjust it by changing the ratios of the major components in a formulation, namely the stabilizer and the protein. For common stabilizers such as disaccharides, an increase in protein concentration with a concomitant decrease in that of the stabilizer will raise Tg. It is unlikely that the stabilizer can be completely eliminated and still retain adequate protein stability in the frozen state. But careful adjustment of their ratio can be made such that a Tg 5–10 °C above the (intended) storage temperature is obtained while maintaining the stabilizer’s cryoprotective effects. More discussion about practical formulation development is provided in part two of this review.
Thawing of Biologics
Although freezing is the principal factor that determines the stability of a biologic when it is stored frozen, the process of thawing deserves some consideration. The major energy requirement goes to provide the latent heat of melting. Although it is simple in principle, the process must be controlled properly to ensure that wall temperatures at the heat-transfer surfaces do not exceed allowable limits for a product. To ensure that the thawed material does not overheat while a remainder is still frozen, the mass should be agitated during processing, which ensures efficient heat transfer and prevents hot spots (43). An agitation rate should be chosen to provide adequate mixing without causing foaming or shaking-induced degradation. An air–water interface is often a site where proteins will unfold.
Apart from the above “macro” phenomenon, thawing can lead to ice recrystallization and annealing. Because of higher surface energy, small ice crystals can melt and preferentially refreeze onto larger crystals, leading to Ostwald ripening effects. In most situations, however, this should not be of any practical concern if the process is carried out reasonably rapidly. Similarly, crystallizable excipients frozen into a metastable state (e.g., mannitol, NaCl) can recrystallize during thawing and have been indirectly implicated in vial breakage during the process (44,45).
Stabilizing Proteins Against the Effects of Freezing
It is apparent from the above discussion that, except for pH changes — and to some extent, phase separation — elimination of stresses is not an option. Cold-denaturation, osmotic stress (cryoconcentration, dessication), and ice-interfacial stress are inevitable consequences of freezing. Formulations and processes therefore must be designed to be cryoprotective against all these factors.
Formulation additives that have been empirically found to be useful as cryoprotectants include sugars, polyhydric alcohols, higher oligosaccharides, amino acids, and surfactants. Other additives include methylamines and lyotropic salts (46,47,48,49). A number of those are proposed to function through the preferential exclusion mechanism (against cold denaturation by lowering the cold denaturation temperature and against osmotic stresses by stabilizing the native state) while surfactants interfere with interactions at the ice interface. Because all stresses are present concurrently, more than one additive is usually required for maximum protection.
A molecular modeling analysis of X-ray diffraction peaks of ice led Varshney, et al. to propose the existence of 2–3 kbars local hydrostatic pressure during freezing (50). Such pressures are sufficient to cause destabilization of protein structures and add an intriguing stress mechanism active during protein freezing.
From a process perspective, within current design limitations the only process parameter available to practitioners is the cooling (heat removal) rate. Because proteins interact with the ice interface and are prone to surface denaturation, a smaller interfacial area with larger ice crystals (a longer freezing time) would be preferable. It would also allow the cryoconcentrated matrix to get closer to its maximally frozen concentration — or nearer to the theoretical Tg (Figure 3). However, a slower rate allows longer exposure to the cryoconcentrated and/or pH-altered medium during the transition between liquid and glassy solid, when there is still enough mobility to damage proteins. Furthermore, it is possible that higher degrees of cryoconcentration could be more damaging to a protein than more dilute matrices would be.
Slower thawing rates can lead to recrystallization, especially in frozen matrices created by rapid cooling, leading to additional perturbation at the ice-liquid interface. Slow thawing also causes longer exposure to cryoconcentrated or pH-altered medium in the transition from a glassy solid to a liquid phase. The basic rule that emerges is that an optimum freezing rate could be defined for each protein, and thawing should generally be as rapid as possible (with agitation). It is likely that proteins with multimeric or multidomain structures are sensitive to stress induced by freezing and thawing than monomeric proteins would be.
Ultimately, real-time stability studies must be conducted to determine the viability of the formulation and storage temperature selected for a given protein. Unlike in the liquid state, viable accelerated conditions are not available. However, studies should be performed both below and above Tg to determine its impact. A limitation to such studies is that the difficulty of simulating full-scale process conditions during development studies. A range of process conditions must be explored and is covered in more detail in part two of this review.
Storage of bulk protein solution in the frozen state is necessary for process economics. Knowledge of the fundamental phenomena described here is critical to the rational design of successful formulations and processes. Part two of this review will provide practical guidance for this purpose.
REFERENCES
1.) Singh, SK 2007. Storage Consideration As Part of the Formulation Development Program for Biologics. Amer. Pharmaceut. Rev. 10:26-33.
2.) Singh, SK. 2009. Best Practices for Formulation and Manufacturing of Biotech Drug Products. BioPharm Int. 22:32-48.
3.) Franks, F 1995. Protein Destabilization at Low Temperatures. Adv. Protein Chem. 46:105-139.
4.) Franks, F 2003. Nucleation of Ice and Its Management in Ecosystems. Phil. Trans. Royal Soc. London, A. 361:557-574.
5.) Bhatnagar, B, RH Bogner, and MJ Pikal. 2007. Protein Stability During Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Technol. 12:505-523.
6.) Cocks, FH, and WE Brower. 1974. Phase Diagram Relationships in Cryobiology. Cryobiol. 11:340-358.
7.) Roos, Y, and M Karel. 1991. Applying State Diagrams to Food Processing and Development. Food Technol. 107 45:66-71.
8.) Matveev, YI, and S Ablett. 2002. Calculation of the Cg′ and Tg′ Interaction Point in the State Diagram of Frozen Solutions. Food Hydrocoll. 16:419-422.
9.) Gayle, FW, FH Cocks, and ML Shepard. 1977. The H2O–NaCl–Sucrose Phase Diagram and Applications in Cryobiology. J. Appl. Chem. Biotechnol. 27:599-607.
10.) Muldrew, K Fuller, BJ, EF and N. 2004.The Water to Ice Transition: Implications for Living CellsLife in the Frozen State, CRC Press, Boca Raton:67-108.
11.) Chaplin, M 2008. Protein Hydration.
12.) Remmele, RL, C Stushnoff, and JF Carpenter. 1997. Real Time In Situ Monitoring of Lysozyme During Lyophilization Using Infrared Spectroscopy: Dehydration Stress in the Presence of Sucrose. Pharm. Res. 14:1548-1555.
13.) Goldberg, RN, N Kishore, and RM Lennen. 2002. Thermodynamic Quantities for the Ionization Reaction of Buffers. J. Phys. Chem. Ref. Data 31:231-370.
14.) Larsen, SS 1973. Studies on Stability of Drugs in Frozen Systems, VI: The Effect of Freezing upon pH for Buffered Aqueous Solutions. Arch. Pharm. Chemi. Sci. Ed. 1:41-53.
15.) Kolhe, P, E Amend, and SK Singh. 2009. Impact of Freezing upon pH of Buffered Solutions and Consequences for Monoclonal Antibody Aggregation. Biotechnol. Prog. submitted.
16.) Cromwell, M 2008.. Opalescence A Clarifying Case Study.
17.) Li, L, A Kantor, A Kantor, and NW. Warne www.wipo.int/pctdb/en/wo.jsp?WO=2007146268.
18.) Taratuta, VG. 1990. Liquid–Liquid Phase Separation of Aqueous Lysozyme Solutions: Effects of pH and Salt Identity. J. Phys. Chem. 94:2140-2144.
19.) Galkin, O. 2002. Liquid–Liquid Separation in Solutions of Normal and Sickle Cell Hemoglobin. Proc. Natl. Acad. Sci. 99:8479-8483.
20.) Thomson, JA. 1987. Binary Liquid Phase Separation and Critical Phenomena in a Protein/Water Solution. Proc. Natl. Acad. Sci. 84:7079-7083.
21.) Shen, VK. 2006. Coarse-Grained Strategy for Modeling Protein Stability in Concentrated Solutions, II: Phase Behavior. Biophys. J. 90:1949-1960.
22.) Strambini, GB, and E Gabellieri. 1996. Proteins in Frozen Solutions: Evidence of Ice-Induced Partial Unfolding. Biophys. J. 70:971-976.
23.) Chang, BS, BS Kendrick, and JF Carpenter. 1996. Surface-Induced Denaturation of Proteins During Freezing and Its Inhibition By Surfactants. J. Pharm. Sci. 85:1325-1330.
24.) Strambini, GB, and M Gonnelli. 2007. Protein Stability in Ice. Biophys. J. 92:2131-2138.
25.) Franks, F. 1990. Water, Temperature and Life. Phil. Trans. Royal Soc. London, B. Biological Sci. 326:517-533.
26.) Franks, F, and RHM Hatley. 1991. Stability of Proteins at Subzero Temperatures: Thermodynamics and Some Ecological Consequences. Pure Appl. Chem. 63:1367-1380.
27.) Jaenicke, R 1990. Protein Structure and Function at Low Temperature. Phil. Trans. Royal Soc. London, B. Biological Sci. 326:535-553.
28.) Privalov, PL 1990. Cold Denaturation of Proteins. Crit. Rev. Biochem. Mol. Biol. 25:281-305.
29.) Becktel, WJ, and JA Schellman. 1987. Protein Stability Curves. Biopolymers. 26:1859-1877.
30.) Hatley, RHM, and F Franks. 1989. The Cold-Induced Denaturation of Lactate Dehydrogenase at Sub-Zero Temperatures in the Absence of Perturbants. FEBS Lett. 257:171-173.
31.) Griko, YV. 1988. Cold Denaturation of Staphylococcal Nuclease. Proc. Natl. Acad. Sci. USA. 85:3343-3347.
32.) Ibarra-Molero, B, GI Makhadatze, and JM Sanchez-Ruiz. 1999. Cold Denaturation of Ubiquitin. Biochim. Biophys. Acta. 1429:384-390.
33.) D’Amico, S, C Gerday, and G Fellert. 2001. Structural Determinants of Cold Adaptation and Stability in a Large Protein. J. Biol. Chem. 276:25791-25796.
34.) Naik, MT, and T-H Huang. 2004. Conformational Stability and Thermodynamic Characterization of the Lipoic Acid Bearing Domain of the Human Mitochondrial Branched Chain (α-Ketoacid Dehydrogenase. Protein Sci. 13:2483-2492.
35.) Tang, X, and MJ Pikal. 2005. The Effect of Stabilizers and Denaturants on the Cold Denaturation Temperatures of Proteins and Implications for Freeze Drying. Pharm. Res. 22:1167-1175.
36.) Schenz, TW 1995. Glass Transitions and Product Stability: An Overview. Food Hydrocoll. 9:307-315.
37.) Piedmonte, DM. 2006. Sorbitol Crystallization Can Lead to Protein Aggregation in the Frozen State. Pharm. Res. 24:136-146.
38.) Kueltzo, LA. 2008. Effect of Solution Conditions, Processing Parameters and Container Materials on Aggregation of a Monoclonal Antibody During Freeze Thawing. J. Pharm. Sci. 97:1801-1812.
39.) Levine, H, and L Slade. 1988a. Thermomechanical Properties of Small Carbohydrate-Water Glasses and Rubbers: Kinetically Metastable Systems at Sub-Zero Temperatures. J. Chem. Soc. Faraday Trans. I. 84:2619-2633.
40.) Levine, H, and L Slade. 1988b. Principles of “Cryostabilization” Technology from Structure/Property Relationships of Carbohydrate/Water Systems: A Review. Cryolett. 9:21-63.
41.) Ablett, S, MJ Izzard, and PJ Lillford. 1992. Differential Scanning Calorimetric Study of Frozen Sucrose and Glycerol Solutions. J. Chem. Soc. Faraday Trans. I.:789-794.
42.) Chang, BS, and CS Randall. 1992. Use of Subambient Thermal Analysis to Optimize Protein Lyophilization. Cryobiol. 29:632-656.
43.) Wisniewski, R, and V Wu Avis, KE and VL. 1996.Large-Scale Freezing and Thawing of Biopharmaceutical ProductsBiotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation, Interpharm Press, Buffalo Grove:7-59.
44.) Williams, NA. 1986. The Effects of Cooling Rate on Solid Phase Transitions and Associated Vial Breakage Occurring in Frozen Mannitol Solutions. PDA J. Parenter. Sci. Technol. 40:135-141.
45.) Milton, N. 2007. Vial Breakage During Freeze-Drying: Crystallization of Sodium Chloride in Sodium Chloride-Sucrose Frozen Aqueous Solutions. J. Pharm. Sci. 96:1848-1853.
46.) Carpenter, JF, and JH Crowe. 1988. The Mechanism of Cryoprotection of Proteins by Solutes. Cryobiol. 25:244-255.
47.) Arakawa, T, Y Kita, and JF Carpenter. 1991. Protein–Solvent Interactions in Pharmaceutical Formulations. Pharm. Res. 8:285-291.
48.) Nema, S, and KE Avis. 1993. Freeze–Thaw Studies of a Model Protein, Lactate Dehydrogenase, in the Presence of Cryoprotectants. J. Parenteral Sci. Technol. 47:76-83.
49.) Carpenter, JF. 1997. Rational Design of Stable Lyophilized Protein Formulations: Some Practical Advice. Pharm. Res. 14:969-975.
50.) Varshney, DB. 2009. Synchrotron X-Ray Diffraction Investigation of the Anomalous Behavior of Ice During Freezing of Aqueous Systems. J. Phys. Chem. B. Lett. 113:6177-6182.
You May Also Like






