Minimizing Variation of Volume Withdrawn from a Vial Drug PackageMinimizing Variation of Volume Withdrawn from a Vial Drug Package
March 1, 2011
Concerns for safety in administration of injectable drug products have escalated in recent years. As a result, scrutiny of administration practices has increased. Pharmaceutical manufacturers are placing greater emphasis on providing the best patient and caregiver experience as well as improving the convenience of drug administration. In fact, many drugs that are regularly administered for chronic conditions are now being offered for at-home preparation and administration. These trends highlight the importance of providing therapies that are not only effective, but also easy and convenient to use. Regardless of treatment location, maintaining a consistent drug dosage to patients is a critical aspect of safety and therapeutic efficacy. To ensure that sufficient drug product is available to administer a full dose, many manufacturers include an certain volume of overfill in each vial. This practice can translate into significant additional expense and greater potential for dosing errors. Inadequate overfill can result in too little drug product available for dosing. Excess overfill is not only costly to manufacturers, but it can leave significant residual product in each vial that could be abused in some way that compromises patient safety and product integrity. PRODUCT FOCUS: INJECTABLES PROCESS FOCUS: FORMULATION, FILL AND FINISH WHO SHOULD READ: PRODUCT DEVELOPMENT, FORMULATIONS, AND BUSINESS DEVELOPMENT KEYWORDS: CONTAINER–CLOSURES, FILLING, DOSING, CLINICAL STUDIES LEVEL: BASIC Reconstitution of freeze-dried drug products is traditionally performed by transferring liquid from a diluent vial into a vial of lyophilized drug through using a disposable needle and syringe. This method creates safety concerns because it increases the risk of needlestick injuries. Innovations in the drug delivery market have provided safety administration systems such as vial adapters that provide for needle-free transfer of liquid for reconstitution and for consistent withdrawal of drug product. A vial adapter is an easy-to-use device that functions by snapping securely over the top of a vial, using a spike to puncture its rubber stopper. Adapters are compatible with Luer-lock and Luer-slip syringes for liquid withdrawal, allowing seamless integration into a clinical setting. The whole process of reconstitution and transfer of drug products can be accomplished without using a needle. These innovative and easy-to-use administration systems with a drug product enable drug manufacturers to differentiate their products in the highly competitive pharmaceutical market — and could ultimately improve patient satisfaction and compliance with dosing regimens. This does not constitute a combination product, however, as described in a recent BPI special report (1). Medimop Medical Projects, Ltd., is a subsidiary of West Pharmaceutical Services, Inc. Medimop manufactures a range of innovative safety and administration systems (including a vial adapter) that aid in reconstitution, mixing, transfer, and delivery of injectable drug products. To better understand industry concerns surrounding consistent dosing, West sponsored a study to compare volumes of liquid withdrawn from a vial using two methods: traditional needle penetration and a vial adapter with spike penetration. Figure 1 is a schematic of the two aspiration methods: the needle penetration method using a standard needle and syringe system on the left and a vial adapter using a spike in its center to puncture the rubber stopper on the right.
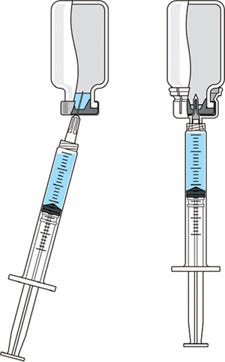
Figure 1:
In certain situations, consistent withdrawal using a vial adapter can help reduce the amount of required overfill. That reduction could provide pharmaceutical manufacturers with cost savings and help make more valuable drug product available to the market. Consider the following scenario. For Example: A drug maker wants to manufacture a drug with a targeted dose of 100 mg. A batch of the product (10 kg) produces 100 vials. If a 30% overfill is required — a costly proposition for high- and even medium-cost drugs because of increased production costs — then an additional 30 mg of drug would need to be dispensed for each vial. So an additional 3 kg of drug would be required to produce the desired batch. Assuming the product costs $100,000/kg, then the total batch drug cost would be $1.3 million. If the required overfill could be reduced to 10% (possibly through use of a vial adapter), then only one additional kilogram of drug would be required to meet the production target. So the total batch cost would be $1.1 million, and the manufacturer could realize a cost savings of $200,000 per batch. Alternatively, the unused 2 kg of drug product no longer required for overfill could be used to make more than 18,000 additional doses, which could then generate additional revenue. That simple example illustrates the potential financial benefit that can be realized by reducing overfill with a vial adapter. Introducting a vial adapter would improve a manufacturer’s cost controls associated with overfill practices, increased amounts of commercially available drug product, and potentially increased patient safety. West’s study compared the withdrawal capabilities of users with various skill levels using a vial adapter and a traditional syringe and needle system. Vial adapters were confirmed to be more accurately and consistently repeatable in all cases. Methods The study evaluated three groups of users and two aspiration methods. The test matrix in Table 1 includes one control group and two experimental groups, which comprised 10 certified nurses and 10 hemophilia patients who had not previously used the vial adapter devices. Those groups were chosen as representatives of clinical (nurses) and at-home (hemophilia patients) administration settings. The control group comprised four experienced laboratory analysts with previous experience using the vial adapters. Table 1: Test subject demographics

Table 1: Test subject demographics ()
Subjects were provided with instructions-for-use (IFU), a video presentation, and verbal instructions on how to aspirate liquid using both methods. They were supplied with vials containing distilled water, plastic disposable Luer-lock syringes, 19-gauge needles, and 13-mm vial adapters with siliconized spikes. Each group completed 100 total aspirations using each method. We determined the volume of aspirated liquid by weighing the syringes before and after each aspiration procedure. Nurses were given a nine-minute time limit to complete all 10 aspirations for each method to more closely simulate their actual working conditions. Results and Conclusions Table 2 shows the average volumes withdrawn for each subject group as well as the standard deviation for all the subjects within each group. Summary statistics for each group (Figures 234 and Tables 345) compare results from the control and experimental groups. Figure 2 indicates that the laboratory analysts’ (control) results were similar for both needle and vial adapter withdrawal methods. Figure 3 indicates a more significant difference between the two methods for the nurse group (experimental). The average volume withdrawn was similar for both methods, but the standard deviation as greater for the method using a needle. The patient group (experimental) demonstrated the greatest variability between methods, which is illustrated by differences in both the mean withdrawal and standard deviation, as well as the range of withdrawal volumes Figure 4, Table 5).
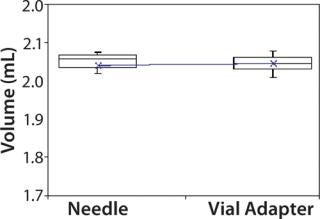
Figure 2: ()
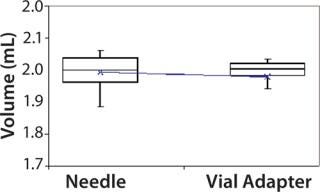
Figure 3: ()
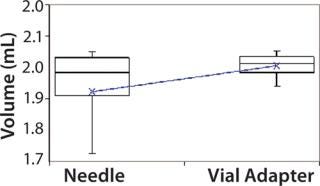
Figure 4: ()
Table 2: Average volume withdrawn and standard deviation for each subject group
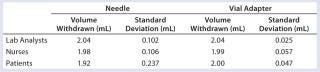
Table 2: Average volume withdrawn and standard deviation for each subject group ()
Table 3: Summary statistics for laboratory analysts’ use of vial adapter and needle withdrawal methods

Table 3: Summary statistics for laboratory analysts’ use of vial adapter and needle withdrawal methods ()
Table 4: Summary statistics for nurses’ use of vial adapter and needle withdrawal methods
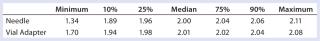
Table 4: Summary statistics for nurses’ use of vial adapter and needle withdrawal methods ()
Table 5: Summary statistics for patients’ use of vial adapter and needle withdrawal methods
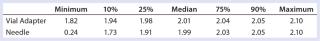
Table 5: Summary statistics for patients’ use of vial adapter and needle withdrawal methods ()
Overall, use of a vial adapter gave greater consistency for liquid withdrawal than use of a traditional needle for both experimental groups. From the data provided, it may also be inferred that the delivered volume of drug product to a patient can be more consistent with a vial adapter than with a needle. To control the volume of drug product delivered to a patient, it is necessary to first confirm that the appropriate volume of liquid is present in a delivery device and that consistency is maintained from dose to dose (as well as user to user). As demonstrated herein, use of a vial adapter is one method for maintaining consistent liquid withdrawal. Because a vial adapter improves dosing consistency, it can also provide an opportunity to reduce overfill associated with variability of dosing across users. Reducing risk of inadequate or excessive overfill protects both patients and manufacturers. For a patient (or nurse), use of a vial adapter provides confidence regarding consistent drug withdrawal. Users can administer accurate doses of drug products with a needle-free device. A pharmaceutical manufacturer can realize benefits of cost contro ls associated with overfill practices and contribute to patient safety by facilitating proper dosing. Biopharmaceutical manufacturers should consider the value of consistent volume withdrawal offered by vial adapters during their drugs’ clinical phases, when planning and management of overfill may be initiated.



About the Author
Author Details Jessica Saggers is a technical account specialist for West Pharmaceutical Services, Inc. 101 Gordon Drive, Lionville, PA 19341; 1-610-594-2900, 1-800-345-9800; [email protected]; www.westpharma.com.
REFERENCES
1.) Rios, MG. 2011. Combination Products for Biotherapeutics: Regulations and Innovations. BioProcess Int. 9:26-35.
You May Also Like






