Integrity Testing Low-Area Filters Using Air–Water Diffusion and an Automatic Integrity TesterIntegrity Testing Low-Area Filters Using Air–Water Diffusion and an Automatic Integrity Tester
Both FDA and EMEA guidelines require integrity testing of filters used in processing sterile solutions such as large- and small-volume parenterals (LVPs and SVPs). The same regulatory agencies also require that corresponding test documentation be included with batch product records.
PRODUCT FOCUS: PARENTERALS
PROCESS FOCUS: DOWNSTREAM PROCESSING, SCALE-UP
WHO SHOULD READ: QA/QC, PROCESS ENGINEERS, AND ANALYTICAL PERSONNEL
KEYWORDS: FILTRATION, INTEGRITY TESTING, VALIDATION, AUTOMATION
LEVEL: BASIC
The function of integrity testing is to determine whether a particular filter is within or outside the validated specifications of its manufacturer’s range for an integral filter to provide a sterile effluent. Successful integrity tests provide users with maximum performance assurance. Nondestructive testing may be performed on filters before and after use. Integrity testing of sterilizing filters before use (after the filter has been sterilized) verifies their integrity before batch processing and prevents the use of nonintegral filters. After a batch has been filtered, integrity testing of sterilizing filters can detect whether their integrity has been compromised during such processing.
Stringent requirements of the biopharmaceutical industry dictate that nondestructive filter integrity testing be performed for each sterilizing application. The most commonly used types of nondestructive integrity tests performed on hydrophilic filters are bubble-point tests (pictured, right) and diffusion tests. Forward-flow tests represent a variation of the diffusion test method.

MILLIPORE CORPORATION (WWW.MILLIPORE.COM)
Background
Low-area filters with sterilizing-grade membranes are typically designed for low-volume sterile filtration applications, often for benchtop work or scaling studies. Using the same membranes as their larger counterparts, these filters should be integrity tested the same way using an automated integrity tester (AIT) or manual referee test. However, filters with low effective filtration areas have inherently low diffusional flow rates. That may present certain challenges when performing a diffusion integrity test of such filters using an AIT:
The claimed capabilities of some AITs do not have sufficient accuracy to test low–flow-rate filters.
An AIT may not have been validated in the test environment for low flow rates with the required degree of accuracy.
Because of difficulties in performing diffusion integrity tests with low-area filters, some manufacturers have not provided specifications for the maximum flow rate of integral low area filters, relying instead on bubble-point specifications alone. However, the physical properties of some membranes make bubble-point testing difficult, which creates a need to perform accurate diffusion tests of low-area filters. The potential issues listed above can be addressed by validating the accuracy of an AIT in the actual test environment using the low-area filters.
Typical AIT Validation
A new automatic integrity tester should be validated before it is used for manufacturing or research. Validation should take place in the environment where the tester will be used, under typical operating conditions, and using the same filters and test methods (e.g., diffusion or bubble point) that will apply in actual use.
When a wide range of filters is tested using an AIT, the recommended procedure is to test only those filters that provide the lowest and highest values for each type of test. If testing is successful, then the AIT is validated to provide accurate and reproducible results across the range of flow rates and pressures represented.
Before testing a new filter on the AIT, it is important to verify that the flow rates and required test pressures for the filter are within the range verified during AIT validation. If, for example, the new filter has a lower flow rate than what was tested during that validation, the AIT should be revalidated for accurate and reproducible diffusion results using the new low–flow-rate filter. The results of the new validation would be appended to the original AIT validation package in a method consistent with relevant documentation policies.

Validating Low-Area Filters on AITs
Validation of a diffusion test for low-area filters will generally be the same as for any other filter. If a new AIT is validated for the first time using low-area filters, one or more of these capsules should be included in the test matrix. If an existing validated AIT is to be used, then the statistical method initially used for determining accuracy and reproducibility should be followed, typically based on an average of three runs with a simultaneous referee measurement.
Potential differences between validating an AIT with small filtration devices and a typical 10-in. filter are addressed below. We used Opticap XL 150, 300, and 600 capsules with Millipore Express membranes for our example.
Accurately Taking a Referee Measurement: The smallest filters in the Opticap XL line (Opticap XL 150 capsules) can have specifications as low as 1 cc/min. This requires a very accurate manual reference meter. The commonly used measurement method using an inverted graduated cylinder is not feasible for this range. Bubble-meters and flow-meters have been found to work well as reference tools. A bubble-meter must have a resolution of at least 0.1 mL, and a flow-meter must have resolution and accuracy of 0.1 mL/min or better. For optimum results, we strongly recommend using a referee instrument with an accuracy of 0.05 mL or 0.05 mL/minute respectively. Using referee devices with that level of sensitivity will enable a meaningful validation.
Programming the Test: All AITs are programmed with default values that may need to be changed to accommodate a new filter type. Data generated during testing show that the accuracy of an AIT diffusion result for an Opticap XL 150, 300, or 600 small-scale capsule (SSC) filter can be greatly improved by increasing the time it allows that filter to stabilize before beginning a diffusion reading. The stabilization time for Opticap XL small-scale capsules should be programmed for 150 seconds (2.5 minutes). All filter manufacturers should provide similar information if their small-area filters require changes in default test programming.

MILLIPORE CORPORATION (WWW.MILLIPORE.COM)
Connecting the Filter: The upstream volume in an AIT can affect a diffusion test. A volume that is too large compared with the filter size reduces the accuracy of filter integrity test measurements. Table 1 lists recommended maximum volumes for diffusion-testing of Opticap XL SSC capsules. Those upstream volumes can be attained by adjusting fixtures to shorten the length of tubing and connectors between AIT and filter.
Table 1: Recommended maximum volumes for diffusion-testing of Opticap XL 150, 300, and 600 small-scale capsules (SSC)
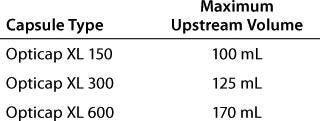
Table 1: Recommended maximum volumes for diffusion-testing of Opticap XL 150, 300, and 600 small-scale capsules (SSC) ()
Using the Integritest 4 AIT Instrument
Millipore’s qualification of its small-scale capsules included multiple diffusion tests using Integritest 4 AIT instruments with simultaneous manual flowmeter readings. The entire set of resulting data can be found in the respective validation guides for Millipore Express filters (1, 2), but a sample is given here in Table 2.
Table 2: Sample qualification data for small-scale capsules
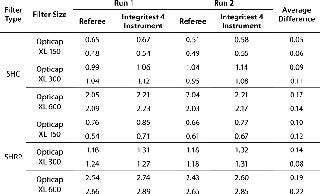
Table 2: Sample qualification data for small-scale capsules ()
The results were generated following guidelines described above. Referee meters had an accuracy of 0.05 mL/min; the test was programmed to stabilize for 150 seconds; and test equipment was set up to reduce upstream volumes to the recommended value listed in the table.
Preliminary capsule testing with Millipore Express SHF and SHR membranes demonstrates that these filters have similar accuracy and repeatability as the already-validated Opticap XL SSCs, which use Millipore Express SHC and SHR with prefilter media. Table 3 contains a sample of those data.
Table 3: Sample qualification data for SHF and SHR filter media
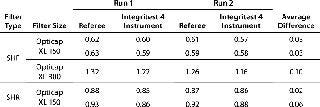
Table 3: Sample qualification data for SHF and SHR filter media ()
The multitude of data generated during our validation of the Opticap XL SSC capsules with Millipore Express media proves the ability of Integritest 4 instruments to perform diffusion tests on such filters with greater accuracy and reproducibility than the minimum claims made for the instrument brand. A validated Integritest 4 instrument thus provides the integrity assurance necessary for sterile operations.
So AITs Can Be Used with Small-Scale Capsules
A number of other integrity testers on the market claim to perform accurate diffusion tests for filters in the Opticap XL SSC flow-rate range. Limited laboratory testing shows that these AITs can produce accurate results (Table 4).
Table 4: Laboratory test results for different AITs
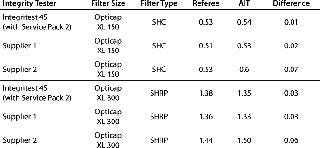
Table 4: Laboratory test results for different AITs ()
Those data indicate that other commonly available AITs can be validated to perform diffusion tests with Opticap XL SSCs. Questions regarding specifics (e.g., test programming, historical low-flow performance) should be directed to their respective manufacturers. Validation may be required to ensure a correlation exists between the AIT measurement of a filter’s flow rate and the measured value obtained from an independent downstream test method.
Tests performed using multiple Integritest 4 instruments and sensitive mass-flow– and bubble-meters have demonstrated that Integritest 4 can be validated to provide accurate diffusion test values for Opticap XL SSCs that use Millipore Express membranes. In addition, the Integritest 4 offers the traceability required for process documentation and the accuracy required for integrity assurance.
REFERENCES
1.) VG1850EN00: Millipore Express SHR Gamma Sterile Validation Guide, Millipore Corporation, Billerica.
2.) VG1012EN00: Millipore Express SHF and SHC Gamma Sterile Validation Guide, Millipore Corporation, Billerica.
You May Also Like





