TFF Membranes for High MAb ConcentrationTFF Membranes for High MAb Concentration
In a typical monoclonal antibody (MAb) purification process, immediately after cell culture and supernatant clarification (its objective being to remove whole cells, cell debris, and particulates), the protein product is typically bound to an affinity chromatography resin and then recovered by elution using a buffer solution. Once recovered, the resulting protein solution is further purified through additional chromatography and virus clearance steps before being concentrated until a final solution is ready for filling and finishing operations.
PRODUCT FOCUS: MONOCLONAL ANTIBODIES
PROCESS FOCUS: DOWNSTREAM PROCESSING
WHO SHOULD READ: PROCESS ENGINEERS, PRODUCT DEVELOPERS, FORMULATORS
KEYWORDS: TFF, UF/DF, BUFFER EXCHANGE, CONCENTRATION
LEVEL: INTERMEDIATE
Membrane-based tangential-flow filtration (TFF) unit operations are used for clarifying, concentrating, and purifying proteins. In TFF, a solution is pumped tangentially along the surface of a membrane filter. Applied pressure forces a portion of the fluid through the membrane to the filtrate side. Particulates and macromolecules that are too large to pass through the membrane pores are retained on the upstream side and swept along by the tangential flow. This feature makes TFF ideal for fine sized-based separations.
MAb downstream processes usually involve one ultrafiltration/diafiltration (UF/DF) step. This is one of the most widely used forms of TFF. It is useful in separation of proteins from buffer components for buffer exchange, desalting, or concentration prior to final product fill and finish. The objective of this step (Figure 1) is to reduce batch volumes and exchange buffers for final formulation.
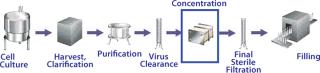
Figure 1: ()
Objectives of a Final UF/DF Step
It is often necessary to administer large amounts of a MAb drug to achieve sufficient therapeutic effects. But dosages are limited by the amount deliverable in a subcutaneous injection volume. Thus, being able to produce a formulation with high MAb concentration in each small injection volume has become a key to success. Reliable, scalable processes must be developed to produce such high concentrations without compromising product quality.
To fulfill the requirements of the biopharmaceutical industry and regulators, it is important to ensure high product purity, quality, and activity; process robustness for high product yield and controlled bioburden and endotoxin levels; and efficient, economical processing.

Figure 1:
MILLIPORE CORPORATION (WWW.MILLIPORE.COM)
Keeping these goals in mind, the main objective of a concentration/diafiltration step in MAb purification is to develop a robust, scalable formulation step that allows the production of formulations with up to 200 mg/mL of the MAb product.
The subsequent related success criteria for the final UF/DF step of MAb purification are thus as follows:
Yield >95% (with the desire to obtain a yield as close as possible to 99.9%)
Retention >99.9 % of the molecule of interest
Highest concentration possible after recovery (around 180 g/L)
A standard UF–DF–UF process time of approximately four hours, not including set-up and cleaning.
Process Parameters
Process Considerations: For the final UF/DF step, a MAb must be first preconcentrated, then diafiltered with a buffer suitable for further injection into patients (e.g., phosphate, histidine, and so on) and then concentrated to its maximum reachable final concentration. Control of the hydraulic parameters (Figure 2) obtained during these successive steps is key to optimizing this step.
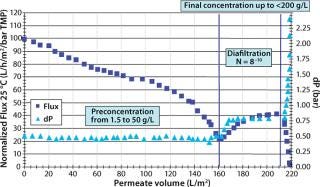
Figure 2: ()
The maximum possible concentration is also called CGEL (the concentration at which a product behaves like a gel on the surface of a membrane). That which can be reached depends on the buffer used and its concentration. For example, a CGEL between 150–200 mg/mL could be reached with a MAb in initial buffer, whereas a CGEL close to 230 mg/mL could be reached in an appropriate final buffer (Figure 3). In other words, the MAb concentration is affected by the buffer used.
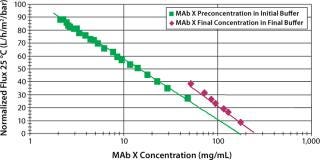
Figure 3: ()
In addition, the appropriate choice of buffer can also have a huge influence on MAb stability over time.
At this step of the purification process for MAbs (usually about 150 kD in size), a Pellicon 3 cassette with a composite regenerated cellulose Ultracel PLC 30-kD C screen membrane was used for each phase. First, ultrafiltration concentrates the MAb from ∼1.5 mg/mL to the optimum concentration for diafiltration (determined during an optimization study). Next, diafiltration is used up to a predefined conductivity criteria to set the MAb in its final buffer. And finally, the MAb is concentrated to a value >200 mg/mL.
Scale-Up Considerations: Table 1 summarizes process parameters obtained during development work (usually performed on 0.1-m2 membrane area) and assessed during pilot work (usually on 0.5–1 m2) and then verified in real cases at manufacturing scale (with ≤1,000 L to be processed).
Table 1: Process parameters
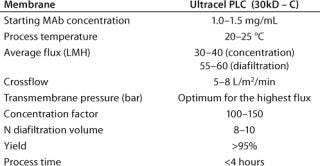
Table 1: Pr ocess parameters ()
Using linearly scalable Pellicon 3 cassettes, when volume increases, membrane area increases linearly, so a process can be performed in a defined period. Table 2 provides several examples of how to control how the process affects the membrane area.
Table 2: Example of scale-up data
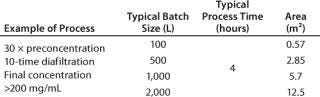
Table 2: Ex ample of scale-up data ()
A Helpful Tool
Operating parameters and membrane performance depend on a variety of factors such as feed-stream composition (purity, protein concentration, and so on), membrane/device selection, and target final formulation. Success criteria for the biopharmaceutical industry were achieved in most studies using Pellicon 3 cassettes with Ultracel PLC 30-kD C screen membrane (Table 3).
Table 3: Success criteria for biopharmaceutical filtration

Table 3: Su ccess criteria for biopharmaceutical filtration ()
In other words, Pellicon 3 cassettes with Ultracel PLC 30-kD C screen membrane can help companies meet the common challenge their researchers are increasingly coming across: to achieve high yields of MAb without any compromise in the final quality or high concentration of the product.
REFERENCES
1.) 2006.Protein Concentration and Diafiltration by Tangential Flow Filtration. Millipore Lit. No. TB032 Rev. C 06/03 03-117, Millipore Corporation, Billerica.
2.) Cheryan, M. 1986.Ultrafiltration Handbook, Technomic Publishing Co., Inc., Pennsylvania.
3.) van Reis, R. 1997. High Performance Tangential Flow Filtration. Biotech. Bioeng. 56:71-82.
4.) van Reis, R. 1997. Linear Scale Ultrafiltration. Biotechnol. Bioeng. 55:737-746.
5.) Zeman, LJ, and AL. Zydney. 1996.Microfiltration and Ultrafiltration: Principles and Applications, Marcel Dekker, New York.
You May Also Like






