I'm Losing Cell Viability and Function at Different Points in My Process, and I Don't Know Why!I'm Losing Cell Viability and Function at Different Points in My Process, and I Don't Know Why!
June 1, 2010
Development of cell and tissue therapies presents bottlenecks in manufacturing process development and scale-up as commercial and academic groups move from small-scale research and development (R&D) to more complex logistics. Often, the simplicity of maintaining cell yield, viability, and function in a laboratory setting cannot be replicated when source tissue and final therapeutic products are subjected to the extended distances and times of actual clinical delivery.
These bottleneck issues have a number of causes. One specific and common cause is suboptimal biopreservation workflow processes and practices. At BioLife Solutions, we often address this particular issue in consultations with current and prospective customers, where we’ve heard the title of this article from a number of people. The following case study — a hypothetical amalgam drawn from our experiences — highlights various biopreservation workflow process optimization steps that can extend stability. This may improve postpreservation viability and functional yield of cellular source materials and final cell-based products.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: MANUFACTURING
WHO SHOULD READ: QUALITY, PROCESS DEVELOPMENT, ANALYTICAL, AND REGULATORY AFFAIRS
KEYWORDS: CRITICAL QUALITY ATTRIBUTES (CQAs), CRITICAL PROCESS PARAMETERS (CPPs), STATISTICAL ANALYSIS, VARIATION, PROCESS OPTIMIZATION, MODELING, BIOPRESERVATION
LEVEL: INTERMEDIATE
Case Study
HairCell Technologies has built on the well recognized understanding that stem cells derived from bone marrow can be used to treat a number of disorders, and the company’s scientists used such cells to develop a proprietary technology that will improve the treatment of hair follicle disease. But during the development of HCT’s technology beyond the R&D stage, several stability-related issues arose that threatened to doom this promising technology as not commercially feasible.
HCT’s phase 1 clinical trial was being conducted in Asia, but the company’s cell processing facility is located in the United States. Development scientists did not realize that such large distances involved in transport of both the source bone marrow and the finished cell therapy product would cause significant yield loss in the materials. They had assumed that an isotonic saline solution would be the natural transport medium for maintenance of cells during ambient shipment. But in fact, there was ~50% loss of cells in the source bone marrow during the first transport stage alone, and isolated stem cells exhibited a significant lag in regrowth.
HCT also observed significant biopreservation issues while manufacturing the two-stage final product. The company banked initially harvested cells as a cryopreserved product to extend its time management profile and allow for patient scheduling convenience. Upon scheduling the final (transplantation) procedure, that frozen product is then thawed at the US facility, cultured for 48 hours in a proprietary activation media, and shipped to Asia for patient application. This not only allows for time management of the final procedure, but it also allows for centralized processing independent of patient location. However, HCT found that cryopreservation of its cell therapy product in the standard cocktail of 10% dimethyl sulfoxide (DMSO) in saline resulted in ~55% cell loss over the 48-hour postthaw activation period. Furthermore, subsequent transport of activated cells in saline resulted in an additional 50% cell loss, with ultimately little evidence of functional utility in patients. HCT’s executives put a hold on the clinical trial, and the company’s primary investor threatened to withdraw continued funding.
HCT faced a bottleneck regarding its source material and final product stability constraints that many involved in cell therapy development might relate to — although perhaps not to the severity of this example. Common nonoptimized process development systems in regenerative medicine tolerate appreciable levels of cell damage/loss, and companies adjust culture times and applications around those limitations. Consider that use of many cryopreserved products often compensates for significant postthaw cell loss by merely increasing the cell concentration or volume of the clinical dose. (But who wants more dead cells injected into his or her body?).
The Challenges: HCT encountered the biopreservation limitations of cellular materials removed from normothermic conditions that provide resources for normal cell function. Biologics begin to degrade once they are removed from a living source organism (cell culture systems and conditions were developed to substitute for natural growth conditions). The sensitivity of cells to the stresses of being removed from their normal conditions is as varied as the different types and functions of the cells within an organism. Consider that kidneys can be preserved for days in a transplant process, but hearts can be preserved only for hours in clinical practice. To slow the degradation of cells and tissues, standard methods for transport or banking involve low temperatures in either hypothermic (2–8 °C) or cryopreservation (most often below –150 °C) ranges. But assuming that the same principles of normothermic biology apply to low-temperature cell models is a common misunderstanding and a critical pitfall encountered in nonoptimized process development.
Some basic considerations should be kept in mind, and not limited to those aspects of low-temperature conditions. Under such conditions, cellular metabolism is reduced, which is advantageous for slowing enzymatic degradation and reducing oxygen demand. But the activity of the adenosine triphosphate (ATP) driven membrane pumps that regulate ionic levels on either side of cell membranes is not maintained under reduced metabolism. Isotonic (extracellular-like) solutions such as saline or culture media were developed to balance cells under normothermic conditions (37 °C, with balanced oxygen and carbon dioxide, nutrients provided, and wastes removed). By contrast, intracellular-like biopreservation solutions — such as HypoThermosol and CryoStor brands from BioLife Solutions (www.biolifesolutions.com) and ViaSpan UW solution from Teva Pharmaceuticals (www.tevausa.com) — were developed to specifically buffer the stresses experienced by cells, tissues, and organs in response to low-temperature preservation. Cells following preservation undergo delayed cell death that is often not fully evident until 24–48 hours postpreservation, with their functional parameters possibly taking even longer to manifest. Figures 1 and 2 show potential variability in biopreservation efficiency.
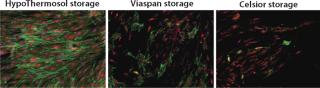
Figure 1: ()
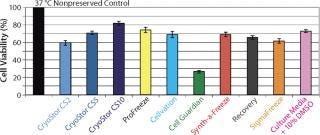
Figure 2: ()
The Solution(s): HCT scientists evaluate several in-house cocktails and commercially available cryopreservation and hypothermic preservation media products, including some containing serum and protein. Commercial cryopreservation solutions include Synth-a-Freeze brand from Cascade Biologics/Invitrogen (www.cascadebio.com), CellVation brand from MP Biomedical (www.mpbio.com), Aedesta brand from Cell Preservation Solutions (www.cellps.com), Cell Freezing Medium solution from Sigma (www.sigmaaldrich.com), and CryoStor brand from BioLife Solutions. Commercial solutions used for hypothermic preservation include saline/HBSS/PBS from multiple suppliers, ViaSpan solution from Teva Pharmaceuticals, Celsior brand from Genzyme (www.genzyme.com), and HypoThermosol solution from BioLife Solutions.
Those solutions are evaluated at HCT for preservation efficacy of cell viability and functional recovery, their quality and regulatory footprints, and their cost effectiveness. On the basis of this evaluation, HCT selects the hypothermic transport and cryopreservation intervals of biopreservation solutions that are specifically formulated for low-temperature biopreservation. In addition to favorable postpreservation viability results, that cells preserved in the chosen solutions demonstrate improved colony forming unit (cfu) measurements, reduced fluctuations in the ratio of proapoptotic/antiapoptotic proteins, and a reduced lag time in the return of a specific target protein activity.
HCT’s regulatory officials feel that the serum-free, protein-free, and fully defined nature of the selected biopreservation media will allow for efficient integration into their existing process, while allowing them the ability to cross-reference their media master files to support clinical trials. HCT’s lengthiest internal discussion entails justification for what appears to be an increased reagent cost. The following factors weigh in favor of switching to the improved preservation media products (similar to considerations presented in Table 1):
costs related to loss of source cellular material
labor costs for “home-brewed” media cocktails
potential reagent costs for serum and/or protein to supplement the performance of previously inadequate media
enablement of global clinical distribution with centralized process logistics.
Table 1: Stability and biopreservation process considerations in defining and selecting optimal conditions for biopreservation steps within a cell- or tissue-based system; these characteristics may provide a useful guideline for processes and reagents.
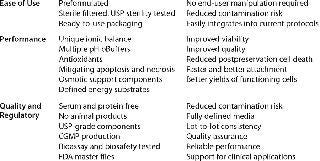
Table 1: Stability and biopreservation process considerations in defining and selecting optimal conditions for biopreservation steps within a cell- or tissue-based system; these characteristics may provide a useful guideline for processes and reagents. ()
HCT business executives find the true cost of improved biopreservation to represent a cost efficient alternative. And the company notes an additional potential advantage to the integration of the new biopreservation media products: Preliminary studies indicate that they may be suitable for use as both a preservation and vehicle solution for their final cell therapy product. This eliminates a costly wash step either before shipment or at a patient’s bedside. HCT is thus able to move forward with its clinical trial. Preliminary results indicate acceptable clinical efficacy of the cell-therapy treatment for hair follicle disease. So the company is able to secure additional funding from its investors.
Disclaimer: HairCell Technologies is a fictional entity. This story is a representative fictionalized amalgam of case studies inspired by actual biopreservation issues encountered by multiple customers of BioLife Solutions, and it is not meant to reflect any particular customer therapy or process. The case study is not meant to endorse a particular biopreservation media product, but rather to highlight potential issues that might be encountered and suggest considerations for evaluating effective biopreservation media products as well as steps toward overcoming process development bottlenecks due to biopreservation limitations. Readers are encouraged to review potential biopreservation shortfalls that might exist in their own current systems and evaluate the efficacy of multiple solutions for potential improvements.
BioPreservation Today (www.biolifesolutions.com/bpt/bpt.htm).
About the Author
Author Details
Aby J. Mathew, PhD, is senior director of strategic relations and senior scientist for BioLife Solutions, 3303 Monte Villa Parkway, Suite 310, Bothell, WA 98021; 1-425-402-1400, fax 1-425-402-1433; [email protected]; www.biolifesolutions.com. Dr. Mathew was part of the founding team of BioLife Solutions and is a codeveloper of its biopreservation media solutions.
REFERENCES
1.) Clarke, DM. 2009. Improved Post-Thaw Recovery of Peripheral Blood Stem/Progenitor Cells Using a Novel Intracellular-Like Cryopreservation Solution. Cytotherapy 11:472-479.
2.) Van Buskirk, RG. 2004. Hypothermic Storage and Cryopreservation: The Issues of Successful Short-Term and Long-Term Preservation of Cells and Tissues. BioProcessing Int. 2:42-49.
3.) Van Buskirk, RG. 2005. Cryopreservation: It’s Not Just About Cell Yield. BioProcess Int. 4.
4.) Baust, JM. 2005. Advances in Media for Cryopreservation and Hypothermic Storage. BioProcess Int. 4:S46-S54.
5.) Mathew, AJ. 2004. Cell Preservation in Reparative and Regenerative Medicine: Evolution of Individualized Solution Composition. Tissue Eng. 10:1662-1671.
You May Also Like






