Biomanufacturing Scenarios: From the Biomanufacturing Technology RoadmapBiomanufacturing Scenarios: From the Biomanufacturing Technology Roadmap
Drug Substance Scenarios
Given the complexity of the biopharmaceutical industry and the increasing diversity of products and companies, it is clear that there will be no “one size fits all” solution to biomanufacturing. Instead, we see a range of biomanufacturing scenarios playing out over the next 10 years. Five high-level scenarios were selected for drug substance manufacturing and two for drug product to cover the full spectrum of process and facility types. Each facility type is associated with a representative set of business conditions reflecting a typical mix of key drivers and business dimensions.

Table 1: Main drug substance facility scenarios with typically associated product types and business drivers
Table 1 summarizes the drug substance scenarios and includes a list of characteristic products and facility features. More detail on each facility type/ scenario follows in the subsections below to provide a vision from which enabling technologies were identified. Since the relative value or impact of a technology will likely depend on the set of business circumstances and facility type, the scenarios are designed to help with visualization and estimating the value of technologies and alternatives. A technology that is determined to have a high impact across multiple scenarios would reinforce its importance to the industry and likely elevate its priority as a technology to be developed further.
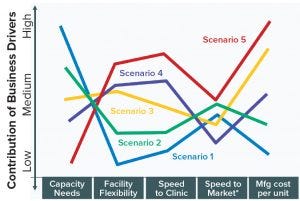
Figure 1: Comparison of business driver profiles for selected scenarios (*speed to market and/or build)
Figure 1 shows the various business profiles for each of the selected scenarios. These business dimensions include capacity requirements and the business drivers that were described in the previous sections: facility flexibility, speed to clinic and to market, quality and cost of manufacturing. For example, Scenario 1 represents a high-volume product where there is a high degree of confidence that the market will support robust sales as long as significant market share can be established. Possibly because treatment is long-term and the dosage is relatively high, there is desire to minimize the cost of production. There may also be significant competition with other players entering the therapeutic space and therefore being the low-cost alternative may be critical to maximizing market share.
As depicted in Figure 1, there is a considerable range in all these potential requirements as drivers. Production outputs can vary from metric tons of a recombinant protein (as has been the case for some blockbuster MAbs) to very small quantities of a drug or preparation intended for a single patient. A facility may be assessed to manufacture proteins, virus or polysaccharides for vaccines, vectors for gene therapy, a specific phenotype for cell therapy or any combination of these. Depending on the market and competitive situation faced by a company, speed to clinic or to market may be paramount. The cost of goods (CoG) may be critical in gaining or maintaining market share and/or in demonstrating superior value.
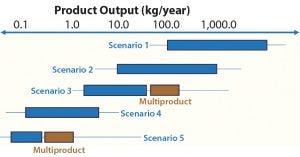
Figure 2: Range of throughputs targeted by facility type
These business driver profiles are relative to each other and represent the trade-offs that are typically expected and progress from one type of profile to another in terms of overall capacity versus cost, flexibility and speed of construction. In general, a given facility type will be most appropriate for a certain range of throughput. However, there are certainly other factors influencing the chosen design of a new facility. Figure 2 shows an approximate range of production rates that would typically be targeted by a given scenario and facility type.
While the scenarios collectively span several orders of magnitude in throughput, they also overlap. Particularly in the designs aimed at multiproduct manufacturing, scale-out options can extend the range of overall production in a given year.
Quantitative computational models provide valuable insight in estimating the value of a technology or process innovation that would otherwise be difficult to predict. The BioSolve analysis tool (from Biopharm Services Ltd.) was used to model the biopharmaceutical manufacturing scenarios. For the first edition of the roadmap, the collaboration team agreed on a set of assumptions for the scenario types defined above that are representative of the typical manufacturing processes in commercial operation today.
Sensitivity parameters were defined for key operations within these scenarios to reflect how the parameters could vary over time with the process improvements that the technology roadmap envisages. Process cost models were built to identify high-cost components and the features of both the process and the typical facility design, and to perform sensitivity analyses to prioritize technology needs in the roadmap. The application of findings from the models to the process technology requirements can be seen in the Process Technologies report.
Manufacturing process modeling is a topic for further consideration in future editions of the roadmap. It offers benefits to all stakeholders in understanding the value and impact of innovation opportunities. For example, a technology that is determined to have a high impact across multiple biomanufacturing scenarios would reinforce its importance to the industry and likely elevate its priority for development and adoption.
Scenario 1 — Large-Scale Stainless Steel Fed Batch: The facility type for this first scenario is a traditional large-scale, stainless steel biomanufacturing facility that has been characteristic of the biopharmaceutical industry for the past 30 years. This scenario is still highly relevant as many companies currently own and operate such facilities and, understandably, wish to continue to leverage their existing assets in the most efficient manner possible.
The facilities were primarily built for the production of recombinant proteins and MAbs with large annual volume requirements. Traditionally, large batch processes have been run both upstream and downstream in these facilities. Furthermore, many were designed based on lower cell culture titers than what is currently being achieved in development and thus the production bioreactor volumes are in the order of 10,000–20,000 L in scale.
The set of business conditions and drivers that would typically accompany a facility of this type would be representative of a company that is in the fortunate position of having a high degree of certainty around the full utilization of a fixed asset of this type. A robust and/or growing product portfolio with a high aggregate certainty of capacity needs, and/or a product with a high certainty of market penetration (such as a follow-on biologic), can justify the investment in a large-capacity facility and the risks associated with a relatively inflexible design.
Thus, these large-scale facilities may remain well suited for business circumstances requiring a very high capacity of protein per year (in the order of a metric ton or more) and seeking a low CoG. Presumably, the company is not encumbered with needing to provide a high degree of regional manufacturing and can therefore centralize manufacturing.
An example of this situation is a contract manufacturing organization that has ready clients and with contracts already in place for significant production quantities. Because of this confidence, the need to supply this additional capacity quickly is not as great due to proactive supply planning and forecasting and the use of existing capacity that will suffice until the new facility or capacity can be built.
For modeling purposes, a conventional MAb process was chosen as a base case. Process assumptions were made that represent a typical process that is currently in commercial operation as a basis for comparing other configurations and improved or alternative technologies. An important question is how should these facilities evolve to accommodate the new business drivers? Another is how does a company minimize or optimize additional investments to adjust to new throughput requirements, improved process conditions and/or yields and recoveries and/or to different product types? For a company considering the construction of a new facility of this type, there may well be design considerations or features that could be incorporated that will allow additional flexibility in the future and/or the adoption of new technologies.
Scenario 2 — Intermediate-Scale Single-Use Perfusion: In this scenario, a facility is designed to take advantage of single-use technologies; e.g., to reduce the capital outlay needed to establish the required capacity and to reduce the requirements for “steam and clean” during operation. However, significant throughput is still required, potentially approaching that of Scenario 1. As a new construction, this facility type may also be enabled with the higher cell-line productivities recently demonstrated on development-phase products. Thus, the high outputs typical of a more conventional stainless steel facility may be possible in smaller reactors operated in perfusion mode. A perfusion-based platform could be a highly productive one and would be particularly well suited to unstable molecules and/or cell lines that may have high media feed requirements.
While single-use bioreactors are now available up to 3,500 L, 2,000 L is currently the largest size in a readily available single-use configuration. Thus, the upstream production bioreactors in this scenario are limited to 2,000 L. To achieve the significant mass output of drug substance, a continuous cell culture is employed. As the base case for this scenario, the downstream portion of the facility is still operated in a batch mode, which is typically the case regardless of whether the upstream process is continuous or not. However, semicontinuous and continuous downstream processes will be explored as part of this study since single-use equipment for protein purification is beginning to emerge on the market.
The set of business drivers that would indicate a facility of this type would be representative of a company that has a need for a moderate to high volume of manufacture with a relatively small product mix. Since a limitation of continuous processing is the relatively longer start-up and shutdown times required, too many product changeovers would not be productive. Instead, to handle additional products or respond to increasing demand, 2,000 L bioreactor trains would need to be added. Scaling out, rather than scaling up, allows a high degree of assurance in technology transfer and product comparability if scale changes can be avoided. Scaling out can also accommodate a lower degree of certainty in product success and demand forecasts. This scenario can also be an advantage from the market launch perspective as a company moves out of late-stage development towards commercial manufacturing, provided that product demand is fairly consistent throughout the year.
By reducing new construction times and costs with the single-use technology, increased flexibility allows a more rapid response to increases in demand that may have been difficult to predict. This approach will likely resound with a smaller company, or one with a smaller product portfolio, that cannot take the same risk on a single product as a larger company with a greater number of assets. Building a traditional facility that then does not achieve full utilization and/or cannot be easily adapted to other needs, may place a financial burden on a smaller organization that could be difficult to sustain.
A conventional MAb process was chosen as the base case for this scenario and for modeling purposes similar to Scenario 1. This allows direct comparison to a large-scale stainless steel bioreactor facility at high throughput (although, to date, not many MAbs are produced using perfusion cell culture). However, this same perfusion platform can also be employed for poorly expressing proteins, enabling acceptable bioreactor volumetric productivities that would otherwise require extremely large batch-mode reactors. Thus, Scenario 2 could also be used to represent a low productivity case, such as the production of a highly complex glycoprotein of low demand (e.g., rare diseases).
Scenario 3 — Intermediate-Scale Multiproduct Single-Use Fed Batch: The facility type in this scenario, similar to Scenario 2, leverages single-use technology. The same 2,000-L scale bioreactors are used since they are the largest that are readily available at present. However, rather than operating them in perfusion (continuous) mode, they are run here as fed-batch reactors, similar to Scenario 1, albeit at a smaller volume. In this scenario, batch mode is employed in the upstream process to enable more products to be accommodated in the same equipment and with shorter cycle times than if they were run in a continuous mode. This design is intended for small- to medium-volume products that individually have demands on the order of kilograms per year but in aggregate could amount to hundreds of kilograms of annual output.
The advantage of a batch process is that variations in process requirements and unit operation sequence may be easier to accommodate for a portfolio of products that do not all use the same process. Longer processes with a larger number of operations, some of which are potentially complex (such as conjugation reactions), would be considerably harder to link together and automate if they were to be run as a continuous process.
A facility of this type offers considerable flexibility both in terms of adapting to different products in a campaign mode but also by allowing scale-out in a similar manner to Scenario 2. Initially, with perhaps just a single train, production campaigns of various durations could be scheduled and sequenced in a way to respond to demand for individual products. If one train can no longer support overall demand, more production trains could be added of the same type and scale. Campaign durations would be relatively short since the bioreactor is sized for overall capacity and not the annualized demand of any one given product. Shorter campaign lengths may be advantageous in terms of the speed of response, lower quality control costs (due to fewer batches for release) and for critical-path clinical production. For this reason, this scenario is becoming increasingly popular for manufacturing products in clinical development by contract manufacturing organizations and innovator companies due to the large number of clinical candidates, low facility fixed costs and reduced changeover times. It is also being considered by larger companies as a more cost-effective way to launch new products, particularly those that are targeting smaller patient populations.
This facility could also more easily accommodate varying cell-line productivities by mixing and matching equipment from multiple trains and pooling intermediates accordingly. Also, with multiple products, each with a limited demand, the advantage of a highly optimized and productive process may not justify the additional development work needed.
Thus, Scenario 3 represents a set of market influences and business drivers requiring an even higher degree of flexibility than Scenarios 1 and 2. A company may be facing a high degree of uncertainty in its product mix and a business calling for the production of several products, potentially with varying processing requirements. The scenario also places a premium on speed by avoiding the relatively longer start-up and shut-down times associated with continuous processing. Similar to Scenario 2, increasing output can be accomplished by scaling out, rather than scaling up, which allows a high degree of assurance in technology transfer and product comparability since scale changes are avoided. There is the added advantage that dramatically different product demands can be accommodated.
The base case process and assumptions for Scenario 3 are described in detail in Appendix B along with all major and minor parameters required to construct such a model. Again, for simplicity, the base case is derived from a typical MAb process similar to Scenarios 1 and 2.
Scenario 4 — Small-Scale <500-L Portable Facility: This scenario represents a very different set of requirements than the previous three. Here, a highly modular construction would be required so that the whole process and any associated required infrastructure could be made portable. The process equipment may even be built into a vehicle to enable rapid transport and deployment to disparate locations. An example of this may be a highly complex biologic, such as a vaccine or allogeneic cell therapy, which requires regional manufacturing. The flexibility of decentralized manufacturing may be a key driver because of regional governmental policies and/or the need to be close to the intended patient population because of product shelf- life and stability, shipping requirements and/or regional emergency responses, such as pandemic flu.
To be portable, by definition, such a facility would be for relatively small production quantities. For products with large overall global demand or increasing demand, more modules could be distributed in strategic locations. To be responsive to growing demand and construction, installation and qualification would have to be fairly rapid. Alternatively, modules could be built ahead in anticipation of deployment and held in reserve, but this presents an investment risk and additional inventory costs.
To make these portable facilities as compact as possible, a highly intensified process would be advantageous to minimize space requirements. Process intensification using continuous process technologies upstream, downstream or both, could be employed. Single-use technology would likely be the design of choice, but not necessarily, if construction can be rapid and the equipment can be dedicated. Stainless steel process equipment would not be as dependent on a steady supply of disposables/consumables, which could be problematic depending on the facility’s location.
In addition to the business drivers already mentioned, this scenario may be associated with a relatively low certainty of approval or market acceptance. Unknown or uncertain requirements for regional manufacturing may also be a factor. This reality would be accommodated by a gradual build out, or in this case a scale out, with the addition of more portable facilities. Thus, a high up-front investment could be avoided by starting with just a few prototypes. More production trains could be added of the same type and scale.
If this scale-out could be accomplished quickly, this application could also be used to satisfy the needs of multiple products by quickly assembling modules with varied process sequences. However, in a continuous mode, this approach would most likely be unsuitable for a large number of products running in parallel. Production cycle times would not have to be short if the equipment is dedicated and can run over extended periods of time and demand is predicable for a given region.
Also, depending on how remote or isolated the location of operation, advanced infrastructure may not be available. In this case, generation of clean water and other utilities may have to be built into the modules. The base case process and assumptions for Scenario 4 are described in detail in Appendix B along with all major and minor parameters required to construct a model.
Scenario 5 — Small-Scale <50-L for Personalized Medicines: In contrast with Scenario 1, with a facility designed to produce one or a small number of products in very high quantities, Scenario 5 represents a facility where many individual preparations are made in parallel. These preparations would typically be made in very small volumes as would be the case for treating individual patients. Some such facilities have already been built and are in operation but they stand at the dawn of personalized medicine. These products may be as complex as those mentioned in Scenario 4 and perhaps even more specialized, such as an autologous cell therapy. Here cells are removed from the patient, brought to a centralized processing facility, expanded and/or treated and then returned to the same patient.
Since a patient has to wait for treatment delivery once their sample has been collected and genotype and/or phenotype has been identified, production cycle times would need to be short. As such, batch processing probably is the most efficient approach due to the small volumes and the simpler set-up and execution compared to continuous parallel processing. The volumes and the bioreactors, if required, would likely be less than one liter. These procedures would likely begin as manual processes but automation may be applicable as the patient count increases. Due to the critical need to segregate patient samples, single-use technology is the only alternative. While automation equipment for vessel and fluid handling could be shared, any surface wetted with a patient-specific cell or gene therapy preparation would have to be completely dedicated.
Cycle time includes shipping the sensitive preparations to and from the processing facility and thus quality release and shipping also need to be done as expediently as possible. Thus, quality control assays testing for safety and potency would have to be designed with this critical-path production schedule and limited product shelf-life in mind.
Clearly, speed of manufacturing is paramount in this scenario. However, speed to build or the ability to expand capacity rapidly may also be important as patients are identified for treatment. Through the use of modular construction, a company could avoid a high up-front investment by starting with a small facility and then expand its footprint as demand increases or add additional facilities that are strategically located. Perhaps modules could be designed as a single unit to be placed in a facility designed for parallel processing. The product mix would probably be relatively small but some processing flexibility may be required to adapt to patient needs.
Specifically for cell therapies, readers are referred to the National Cell Manufacturing Consortium (NCMC) Cell Therapy Roadmap: www.cellmanufacturingusa. org/sites/default/files/NCMC_Roadmap_021816_high_ res-2.pdf. In the future however, synergies regarding this facility type and associated new technologies with other modes of biomanufacturing should be explored. Also, while cost may be less of an issue in these early therapies, at some point the COGS will have to be addressed. This would include understanding what overall design aspects most impact the COGS, including how the final product is shipped to the patient and administered.
At some point, a whole new paradigm may be considered for delivering a therapy to a patient. Currently, the therapeutic preparations are made in a centralized or regional facility. However, manufacture at bedside could be envisaged using a modular and mobile approach. However, a number of questions would have to be addressed, such as how do E2E costs compare with a more centralized facility? And could this be made highly portable and brought to a patient’s bedside for deployment or would it have to be set up in a hospital permanently?
Drug Product Scenarios
The primary scope of this first roadmap edition is drug substance. This section provides a high-level view of the scenarios impacting drug product, with metrics and initial considerations to deliver the anticipated 10-year targets. This is a topic for more detailed consideration in future roadmap editions.
From a drug product perspective, there are two base scenarios: “low volume” and “high volume,” which are determined by the overall production volumes of the particular product in terms of annual throughput. Devices and combination products are considered out of scope for this first edition of the technology roadmap. In general, technologies for drug product are primarily determined by the product presentation (the primary container and container closure system rather than the therapeutic modality). As a standard, fill–finish processes are operated with minimal human interface, with isolator technology and restricted access barrier systems both considered equally qualified technical solutions for ensuring sterility.
As is the current conventional approach, it is assumed the bulk drug substance is received at the drug product manufacturing site as pre-formulated, or formulated, and ready to fill. It is also assumed that the product has extended room temperature stability, such as formulations of mammalian cell culture (e.g., Chinese hamster ovary (CHO) cells) and E. coli-derived active pharmaceutical ingredients, as well as vaccines.
This will significantly reduce the complexity of logistics of drug substance, drug product and all drug product manufacturing operations. Although not specifically addressed, personalized medicine can be considered to be the extreme version of the “low volume” scenario, although there may be some additional challenges that are not specifically addressed in this version of the technology roadmap.
Low-Volume Drug Product Manufacturing Scenario: Low-volume products are expected to become an essential part of the industry’s portfolio in the next 5–10 years. While small in overall batch size, many of these products can be considered high value hence any loss of product during manufacturing operation has a considerable business impact. The “right first time” quality is of utmost importance. Today’s relatively high reject rates, often due to the insufficient quality of raw materials (particularly primary packaging) or processing steps, are deemed not acceptable. Continuous improvement efforts in the coming years will drive the yield up to >99%. Future portfolio needs will require faster product changeover, format changes and product release times. It is expected that drug product manufacturing processes will be further simplified (e.g., by consistently using pre-sterilized ready-to-fill primary packaging) and hence, avoiding in-house washing and sterilization steps.
A high degree of automation further reduces operator interference and testing requirements. As an example, consider the time and resources currently spent for glove integrity testing for isolators, or the full-time equivalent needs for processing in classified areas, which still needs operators and exhibits very complex operating procedures. Further, the broader use of single-use/ disposable components instead of multi-use stainless steel will reduce cleaning and validation needs. Highly flexible multiproduct/multipresentation lines (“modular filling lines”) will become a new industry standard. The metrics and 5–10-year targets for the low-volume DP scenario are shown in Table 2.
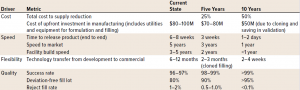
Table 2: Drug product metrics for low-volume scenario
High-Volume Drug Product Manufacturing Scenario: Besides the trend for more personalized medicines that require less overall manufacturing volume, high-volume products are still considered part of industry’s future portfolio, especially when dealing with mass-vaccination products. These highthroughput production lines still require significant upfront investment but prices will fall due to industry standards because of less customized technical user requirements. This will also benefit the speed of building a new facility.
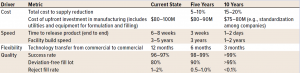
Table 3: Drug product metrics for high-volume scenario
The metrics and 5–10-year targets for the highvolume DP scenario are shown in Table 3. More specifics on drug-product–related needs can be found in the In-Line Monitoring and Real-Time Release, and Modular and Mobile reports.
At the time of first publication, Rick Lu was with AstraZeneca; Elania Clark was with Biogen; Stewart McNaull was with Fujifilm Diosynth Biotechnologies; Charles Heffernan was with GlaxoSmithKline; Thomas Ryll was with ImmunoGen; Ranjit Thakur and Stefan Merkle were with Janssen; Rajesh Beri was with Lonza; Jonathan Souquet and Beth Junker were with Merck; Joris Smets and Paul McCormac were with Pfizer; Oliver Stauch and Tina Larson were with Roche; Bert Frohlich was with Shire; Steve Jones, Paul Ilott, Linda Wilson, and Clare Simpson were with the Bio-Phorum Operations Group; and Alan Calleja was with Biopharm Services.
You May Also Like






