PACE™ Cell TechnologyPACE™ Cell Technology
July 1, 2008

Figure 1.
The costs of producing monoclonal antibodies (MAbs) from mammalian cells are extremely high. Producing 500 kg/year of a MAb can run $200,000,000/year or more. Great effort has been made by the biopharmaceutical industry to reduce these costs. Companies have improved or expanded their existing infrastructures for MAb manufacturing, optimized media formulations and feeds, and/or genetically engineered production cell lines, with the current industry goal of 10 g/L titers and productivities of 100 picograms/cell/day. A twofold increase in volumetric productivity can easily be expected to provide savings of 20% or more in costs of goods sold. Increases of this magnitude translate to costs savings in upstream biopharmaceutical production amounting to 6–10% of total sales.
The CDI Bioscience (CDI) solution to improving upstream yields is a patented and innovative technology to increase the yield of bioproducts manufactured in cellular systems. Genetic engineering is used to introduce factors that, when activated, trigger a phenotype change in production cells, shifting them from a replicative to production state (RP Shift®). CDI has created PACE™ cells, a platform cell line and expression vector system competent with the RP Shift® technology known as derived from DG44 CHO cells. End-users can genetically engineer any recombinant cDNA product into PACE™ cells using the expression vector of choice, or our new PACE™ vector, specifically designed to upregulate gene expression during the RP Shift® process.
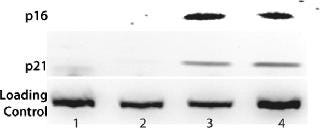
Figure 1. ()
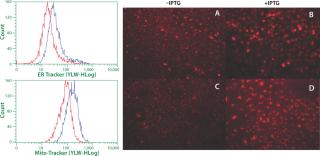
Figure 2. ()
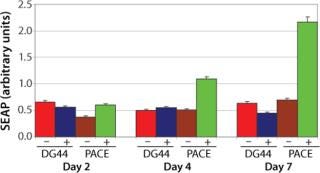
Figure 3. ()
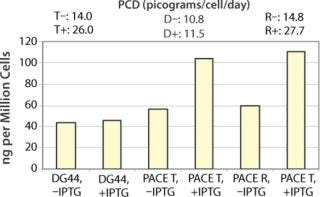
Figure 4. ()
Phenotype modifying factors, active domains of INK4A p16 and Cip/Kip p21 proteins, are regulated by the lactose operon repressor protein, which binds to operator elements in the promoter of the RP Shift® plasmids. Addition of a galactoside inducer removes repressor from the operator, allowing expression of phenotype triggering factors. PACE™ cells undergoing the RP Shift® process increase in size, expand their endoplasmic reticulum, multiply their levels of mitochondria, and increase synthesis and secretion of protein. CDI has routinely achieved three- to sevenfold increases in protein production from commercial cell lines with titers >5 g/L and productivities >100 PCD.
CDI has prepared a PACE™CHO DG44 cellular clone displaying high levels of endoplasmic reticulum and mitochondria with induced production of the enzyme secretable alkaline phosphatase and IgG. CDI is creating master and working cell banks and performing all quality control tests to ensure stability and sterility of the cell line. The PACE™CHO DG44 cell line will be available in August 2008.
You May Also Like






