A Novel Dry-Format Supplement for CHO CellsA Novel Dry-Format Supplement for CHO Cells
April 1, 2011
The biotechnology industry is continually looking for new methods of improving titer of biotherapeutic proteins. Numerous reports show that nutrient supplementation improves productivity several-fold (1,2). Maintaining cells in a viable and productive condition is the ultimate goal and generally involves adding small volumes of concentrated nutrients to cell cultures. Important parameters for designing a nutrient supplement include ease of use, operator and site safety, and product storage footprint at a manufacturing facility. Traditionally, these supplements come as concentrated liquids, which can present logistic issues when large quantities of liquid must be shipped to and stored at a manufacturing site. In addition, highly concentrated components may cause precipitates to form in storage containers during long-term storage.
Dry-format nutrient supplements (e.g., milled powders) minimize many shipping and storage problems but may have other issues, including extended mixing times for complete solubilization, the need for separate component addition, and homogeneity of minor components such as trace elements. Certain commercial dry-format supplements must be dissolved at warm temperatures to get all components in solution. Some vendors’ supplements must be used the same day as they are reconstituted to prevent component precipitation, which presents numerous logistic issues with bandwidth and consistency.
PRODUCT FOCUS: BIOTHERAPEUTICS FROM CHO CELL CULTURE,
PROCESS FOCUS: PRODUCTION
WHO SHOULD READ: PROCESS AND CELL CULTURE ENGINEERS
KEYWORDS: CULTURE MEDIA, NUTRIENTS, SUPPLEMENTS, FLUID-BED GRANULATION
LEVEL: INTRODUCTION
Advanced Dry-Format Media
AGT (advanced granulation technology, Life Technologies Corporation) is a process for producing complete dry-format cell culture media with improved characteristics over milled powders (3). Advantages of a granulated format include rapid wetting and absence of dust. Our technology has recently been implemented in creation of complete dry-format feeds. We applied this technology to a concentrated feed supplement formulation (CHO CD EfficientFeed B AGT supplement) to improve utility for large-scale biomanufacturing. The reconstituted granulated powder maintains efficacy up to six months when stored at appropriate conditions. The product exhibits all previously listed advantages while boosting peak viable cell density and productivity. Biological performance indicators of the granulated format supplement were comparable with those observed for concentrated liquid supplement. We consistently demonstrated fourfold increase in cell densities over nonfed-batch cultures. Benefits in ease of use, growth, and productivity improvements make AGT supplement an advantage for large-scale manufacturing processes.
Consistent Lot Manufacturability
CHO CD EfficientFeed B nutrient supplement was first provided commercially as a concentrated liquid. When converting liquid media and feeds to a dry format such as AGT, it is imperative to show equivalency of performance. We tested the equivalency of three 50-kg current good manufacturing practice (CGMP) AGT lots, a pilot 2-kg AGT lot, and liquid CHO CD EfficientFeed B. We used a recombinant CHO DG44 cell line expressing erythropoietin (EPO). Cells were recovered from cryopreservation in CD CHO medium supplemented with 8 mM/l-glutamine. We incubated all cultures at 37 °C and 8% CO2 and shook them at 125 rpm. We seeded cultures (40 mL) at 3 × 105 viable cells/mL and grew them in 250-mL shake flasks with caps loosened to permit gas exchange. Feeding protocol was identical for all five culture conditions; 10% v/v was added on days 2, 4, 6, and 8 without a glucose or glutamine feed. An unfed culture served as a baseline for growth without supplementation. We measured growth and viability on days 2–12 using the Vi-Cell cell counting system (Beckman Coulter, Inc.). EPO concentrations were determined on days 3, 6, 9, and 12 using a Forte’Bio Octet (Forte’Bio, Inc.) using streptavidin sensors. Figures 1 and 2 show the results.

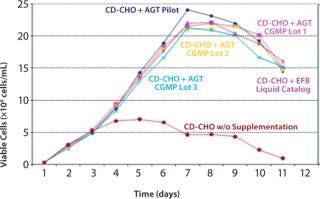
Figure 1: ()
A Complete Feed Supplement
Life Technologies developed a dry-format, granulated, complete feed supplement from the concentrated liquid formulation. This supplement exhibited rapid dissolution as a result of enhanced hydration characteristics. Our experiments showed superior growth and titer in CHO cells compared with basal media without supplementation and compared with other commercially available supplements. We also demonstrated consistency of performance with three CGMP lots.
REFERENCES
1.) Fike, R 2009. Nutrient Supplementation Strategies for Biopharmaceutical Production: Feeding for Optimal Recombinant Protein Production. BioProcess Int. 7:46-52.
2.) Fike, R 2009. Nutrient Supplementation Strategies for Biopharmaceutical Production: Identifying a Formulation. BioProcess Int. 7:44-51.
3.) Radominski, R. 2001. Production-Scale Qualification of a Novel Cell Culture Medium Format. BioPharm. 14:34-39.
You May Also Like






