Controlling Glycosylation in Fusion Protein Manufacturing to Generate Potent BiobettersControlling Glycosylation in Fusion Protein Manufacturing to Generate Potent Biobetters
September 19, 2017
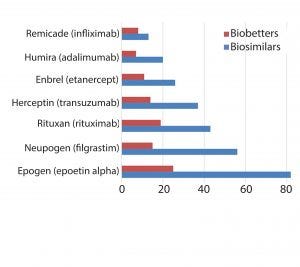
Figure 1: Innovator products and their biosimilar and biobetter variants
The pipelines of pharmaceutical companies are full of biological drugs. Many of them are innovative therapeutic proteins, but a growing number represent biosimilars and biobetters (Figure 1) (1). Biobetters typically are defined as being “based on innovative biologics but with improved properties” (2). Their development benefits from known therapeutic approaches and mechanisms of action resulting in low risk, fast paths to the clinic and thus lower costs. Superiority is achieved through extended half-life (t1/2), improved efficacy, and reduced immunogenicity or toxicity (3). Some desirable improvements such as enhanced pharmacokinetics can be obtained by genetically connecting additional domains to the protein of interest (4). For instance, several half-life improved fusion proteins recently have received market approval as biobetters for coagulation factor IX (FIX) or glucagon-like peptide 1 (GLP-1) (Table 1).

Table 1: Approved biobetter fusion proteins
Glycosylation
Alternatively, a modification of glycosylation can be applied to enhance circulation times. Glycosylation is a posttranslational modification in eukaryotic cells that affects a number of biologically relevant functions such as pharmacokinetics, pharmacodynamics, and immunogenicity. Therefore, it is imperative to control glycosylation properly during a manufacturing process. Glycosylated proteins contain carbohydrates either N-linked to the nitrogen in asparagine side chains or O-linked to the oxygen in serine or threonine. During the passage through the endoplasmic reticulum (ER) and the Golgi apparatus, N-linked glycans are attached in a three-step process. Precursor oligosaccharides are synthesized, then transferred to asparagines as an activated polymer block, and finally enzymatically trimmed. This step is quite sensitive to culture conditions, and therefore N-glycans require more thorough analysis. The process can distinguish among glycans with high mannose content, complex oligosaccharides with a high degree of sialic acid, and hybrid versions (Figure 2).
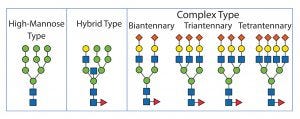
Figure 2: N-linked glycosylation types
N-linked glycosylation to Asn requires the recognition motif of asparagine-X-serine-threonine (Asn-X-Ser/Thr), where X can be any amino acid except proline. O-linked glycosylation does not require a consensus sequence, but rather relies more on the secondary structure and accessibility of Ser or Thr. Furthermore, the O-linked sugars are attached as single monosaccharides sequentially in the Golgi. Subsequent modification by glycosyltransferases results in the mature O-glycan.
The terminal sugar in O-linked oligosaccharides typically is sialic acid. There are several variants of sialic acid, but only NANA (N-acetylneuraminic acid) has a positive impact on half-life. Note that the final structure of N- or O-linked glycans is not related to protein sequence, but rather depends on the correct three-dimensional structure as well as on the expression of necessary enzymes and the availability of sugar substrates in the ER and Golgi (5). That ultimately leads to heterogeneity in the glycan structure that increases the downstream processing (DSP) and analytic efforts to produce a homogeneous and well-characterized drug substance.
Effects of Glycosylation
Half-life is influenced by glycosylation. The addition of large oligosaccharide structures can increase the hydrodynamic radius of glycoproteins substantially, thus reducing the level of renal filtration, which is size dependent. The complete capping of galactose residues by sialic acid fulfills three functions: repulsion in glomerulate filtration because of negative charges, prevention of the elimination through asialo-glycoprotein receptor binding, and increase of the hydrodynamic radius and thus greater protection from size-dependent filtration (6). Because sialic acid is present on N-linked or O-linked glycans, both variants can help extending half-life significantly.
For instance, the circulation time of etanercept has been directly correlated to the degree of sialylation at the TNFα receptor domain (7). The same effect was demonstrated for the sialylation of O-linked glycans of a BR3-Fc-fusion protein as well as N-linked glycans in the IL-23R-Fc-fusion protein, CTLA4-Ig fusion protein, LFA3-Fc-fusion protein, and TNFαRI-Fc-fusion protein (8).
Glycosylation also can reduce half-life. For cases with high mannose content, protein is quickly cleared from the blood stream through mannose receptors on macrophages and hepatocytes. Specifically, the M5 variant species is removed the fastest (9). On the other hand, glycoproteins with high mannose content can be used for targeting therapeutic proteins to specific cell types. Enzyme replacement therapy for lysosomal storage diseases relies on that principle, specifically addressing macrophages. The first enzyme drugs had to be chemically remodeled, but now the right choice of expression system can deliver glycoproteins with high mannose content (10).
Antibody-dependent cell-mediated cytotoxicity (ADCC) is part of the adaptive immune response and describes the attack of immune cells on target cells whose surface antigens are bound by antibodies. The connection between the antibody and the effector cells depends on the presence of a certain N-linked glycosylation at position Asn297 in the heavy chain of the CH2 domain of IgG — the region where the immune globulin interacts with Fcγ receptors. The absence of glycosylation at this position decreases the binding affinity to FcγRI and completely abolishes binding to FcγRII and FcγRIII. However, if only fucose is removed from glycan, then antibodies show an increased affinity to FcγRIIIa — primarily because of the absence of steric hindrance between carbohydrates on the antibody and receptor (11).
Terminal galactose is a key element in complement-dependent cytotoxicity (CDC) activity. CDC increases proportionally to the galactose content in the heavy chain of rituximab, whereas the affinity to C1q has been shown to increase twofold from G0 to G2 sugars (12). Shen et al. described a biobetter version of etanercept that exerted improved binding to membrane bound TNFα and that exhibited ADCC activity as a new mode of action (13). That discovery could lead to further improved receptor traps and other glycoengineered Fc-domain containing fusion proteins without changing the amino acid sequence.
Toxicity: In the case of fusions of microbial or plant toxins to targeting moieties such as single-chain antibodies to generate immunotoxins, studies have shown that the nonglycosylated diphtheria toxin is 12-fold more toxic than the native version with N-linked glycans. Therefore, the asparagines in the recognition sequence were replaced by alanine to obtain the toxic fusion protein (14).
Immunogenicity: Large carbohydrate structures added during glycosylation potentially can shield immunogenic peptide epitopes from recognition by the immune system. Nonhuman or nonmammalian glycans such as high-mannose N-glycans or N-glycolylneuraminic acid (Neu5Gc) and galactose-α1,3-galactose (α-Gal), however, can cause immune reactions. Structures of α-Gal epitopes or Neu5Gc specifically have been described as targets for antidrug antibodies (ADAs) (15).
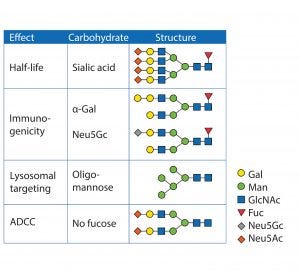
Figure 3: Glycan structures and their biological effect
Solubility and Stability: Hydrophobic patches of proteins provide a shielding effect of glycans. A high hydrophilic content of sugar structures increases solubility. It also contributes to thermal stability because proteins typically tend to aggregate through hydrophobic patches after heat-induced unfolding (16). Another protective function of glycan structures is the steric hindrance of proteases, thus limiting degradation (17). Figure 3 shows typical glycan structures and their functions.
ControllinG Glycosylation
Molecule Design: The first step in manipulating glycan content is through the adaptation of the amino acid sequence. Darbepoetin alfa (Aranesp) is a hyperglycosylated analogue of human erythropoietin (EPO) containing five (instead of three) N-linked glycosylation sites. This new variant exhibited a lower pI (3.3 instead of 4.0), a larger molecular weight (37 kDa instead of 30 kDa), and an increased carbohydrate content (51% instead of 40%) than the older variant. The half-life was significantly increased from 8.5 h to 26.3 h. Although five amino acids were changed, the number of ADAs did not increase, probably as a result of the shielding of the novel epitopes by the glycans (18).
A similar case is the glycoengineering of blood coagulation factor VIIa (FVIIa) (BAY 86-6150). Two more N-linked glycan attachment sites have been introduced in the original sequence of FVIIa. That modification improved the half-life two to threefold over the half-life of wild-type FVIIa (19).
Peptide sequences with multiple glycosylation sites can be added. A frequently used building block for this concept is the C-terminal peptide (CTP) of the human chorionic gonadotropin (hCG)-b-subunit. That domain contains four O-linked oligosaccharide recognition sites. Despite its name, CTP can be fused to the N- or C-terminus or both termini. In one example, one CTP unit was fused to the amino terminus, and two CTP domains were added to the carboxyl end of EPO. The molecular weight increased to 57 kDa due to 84 amino acids from the CTP and a huge amount of O-linked oligosaccharides. The half-life increased threefold, and no immune response was triggered (20). The same principle was applied for FVIIa by attaching CTP at the C-terminus. This modification resulted in a three- to fivefold half-live improvement for FVIIa-CTP (MOD-5014) (21).
An alternative method uses the incorporation of polysialic acid (PSA). This was exemplified by fusing the PSA carrier domain of NCAM to the C-terminus of an scFv and expressing the construct in transgenic HEK293 cells containing polysialyltransferase. The polysialylation increased the hydrodynamic radius threefold, resulting in a 12-fold improvement of the circulation half-life (22).
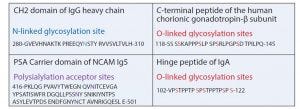
Figure 4: Typical building blocks for fusion proteins containing glycosylation sites
Frequently, short peptide fragments serve as flexible linkers between the domains of fusion proteins. Serine in glycine-serine (Gly-Ser) linkers is a target for O-linked xylolysation. This glycan can be immunogenic, so the motif was changed by replacing one Gly neighboring the Ser by proline (Pro) (23). In another case, an O-linked glycosylation site was removed to restore proper activity of an anti-IL17a peptide in a fusion protein (24). In one study of a hinge peptide of IgA, researchers demonstrated that an increase of O-linked glycosylation reduces the flexibility and restricts the conformation of the linker (25). However, the introduction of glycosylation sites into linker domains supports the proper folding of complicated fusion proteins because it slows down protein processing and includes intracellular quality control, thus resulting in stable proteins. Figure 4 shows typical building blocks.

Table 2: Differences in glycan patterns of frequently used host cells
Cell Line and Strain Engineering: The next level of glycosylation control is the choice of host cell line. Typical production hosts such as Chinese hamster ovary (CHO), human embryonic kidney (HEK293), PER.C6, NS0, and SP2/0 cells significantly differ in their glycan patterns (Table 2). CHO cells are used widely for manufacturing proteins because they deliver human-type glycans. However, they are unable to generate α-2,6-sialylation and α-1,3/4-fucosylation and even attach some nonhuman and immunogenic glycans such as Neu5Gc and α-Gal. Figure 5 shows host-cell differences.
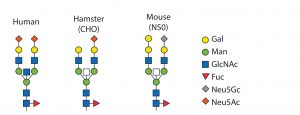
Figure 5: Glycan patterns of different cell types
Some shortcomings of the hosts can be eliminated by strain engineering, which is either addition or removal of glycosyltransferases. Enzymes not present in humans such as α-1,3-galacto-syltransferase or CMP-Neu5Ac hydroxylase particularly should be knocked out. Elimination of α-1,6-fucosyltransferase (FUT8) activity in CHO cells leads to absence of fucose in glycans. That is beneficial for antibodies and Fc-fusion proteins to enhance their ADCC abilities (26).
ADCC also can be improved through the introduction of b1,4-mannosyl-glycoprotein 4-b-N-acetylglucosaminyl-transferase III (GnTIII), which blocks fucosylation and causes bisecting antennas. ADCC functionality also can be enhanced by coexpressing microbial sialidase A, which cleaves the terminal sialic acid residues (27). Elimination of sialidase activity in CHO cells by RNA interference, however, increased the overall amount of sialic acid (28). The ability of CHO cells to generate α-2,6 sialylation can be restored by overexpressing α-2,6-sialyltransferase (29). Sialylation also can be increased by introducing a gene such as b1,4-galactosyltransferase that generates the terminal galactose to which then sialic acid is then transferred (30).
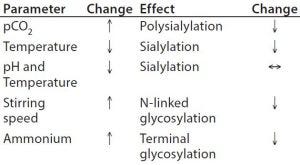
Table 3: Process parameters influencing glycosylation
Upstream Processing (USP): Glycan structures can be influenced by growth conditions in a bioreactor. Typical physicochemical process parameters having an effect are pH, temperature, CO2, and ammonium ion concentration (Table 3). Titration of osmolality can be used to steer the degree of fucosylation. Important enzymatic cofactors should be present in sufficient quantity. Specifically, manganese and iron facilitate increased glycosylation. Other feed or media components such as the typical carbon source glucose, its replacement by galactose, mannose, or other glycosylation precursors have an impact on glycan structure, including uridine diphosphate–N-acetylglucosamine (UDP–GlcNAc), uridine diphosphate–galactose (UDP–Gal), and cytidine monophosphate–sialic acid (CMP– SA).
Additives in the medium also can steer glycosylation during upstream processing. The immunogenic content of Neu5Gc in a fusion protein could be reduced by 50% with the addition of sodium butyrate. Furthermore, a late temperature shift and a pH control by NaOH can support low Neu5Gc levels (31). One study has shown that activation of the glucocorticoid receptor by hydrocortisone improved the degree of sialylation of an Fc-fusion protein dependent on dose (32).
Besides glycoengineering at the gene level, unwanted enzymatic activity can be blocked by specific inhibitors. For instance, fucosyltransferases can be blocked by fluorinated fucose analogs (33) or certain reactive dyes (34). Sialidases have been addressed by dextran sulfate, and mannosidase 1 inhibitors are used to generate high-mannose structures (35).
Downstream Processing (DSP) typically starts with a cell-free supernatant. Ideally, to prevent the release of intracellular glycosidases — particularly sialidases, which could decrease sialic acid content — cell lysis does not occur
during a harvest procedure. Despite the many possibilities of controlling glycosylation during USP, usually some heterogeneity remains. Those different isoforms can be separated according to charge variations with shallow gradients on anion-exchanger resins. The negative charges are contributed primarily by sialic acids. Highly charged forms also can be distinguished from less intensely glycosylated variants because of their individual degree of hydrophobicity. That principle was applied with a highly glycosylated Fc-fusion protein that could be purified in a combination of ion-exchange and hydrophobic-interaction chromatography (HIC) (36).
In general, glycans can be much more hydrophilic than Asn without conjugated oligosaccharides, therefore binding more tightly to HIC resins (37). In some cases, hydroxyapatite (HTP) has been used to discriminate between glycan variations. For example, six isoforms of α1-acid glycoprotein (AAG) could be separated with HTP and a phosphate gradient at low pH (38). The resin repertoire for analytical purposes is even larger also containing affinity ligands with high specificity such as lectins or titanium dioxide (39).
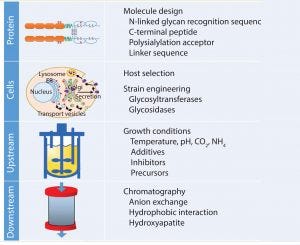
Figure 6: Controlling glycosylation
Moving Forward: Progress and Optimization
In the past few decades, great progress in understanding glycosylation mechanisms and their effects has been achieved. Biobetters in general have benefited from the various approaches to optimize glycans (Figure 6). Strategies focus on extension of circulation half-life, and a trend toward improved functionalities can be observed. In the case of fusion proteins, we are likely to see enhanced Fc-fusion proteins with a caveat remaining: Glycoengineering in that context competes with amino acid exchanges that could deliver similar effects. Competition in the biobetters industry is increasing (Figure 1 and Table 1), indicating that different molecule versions can address the same market and suffer from little differentiation potential. In many cases, glycosylation is a critical quality attribute requiring tight control of parameters. Because of the complexity and multiple possibilities to optimize glycoproteins, manufacturers should organize all efforts according to quality by design principles to enable a systematic fine tuning to achieve desired effects.
References
1 Dorey E. How the Biologics Landscape Is Evolving. Clin. Pharm. 6(9) 2014: 1–6.
2 Strohl WR. Fusion Proteins for Half-Life Extension of Biologics As a Strategy to Make Biobetters BioDrugs 29(4) 2015: 215–239.
3 Sandeep V, et al. Biobetters: The Better Biologics and Their Regulatory Overview. Int. J. Drug Regul. Aff. 4(1) 2016: 13–20.
4 Kontermann RE. Strategies for Extended Serum Half-Life of Protein Therapeutics. Curr. Opin. Biotechnol. 22(6) 2011: 868–876.
5 Borka K, et al. The Expression of Sialyltransferases Is Regulated By the Bioavailability and Biosynthesis of Sialic Acids. Gene Expr. Patterns 23–24, 2017: 52–58.
6 Morell AG, et al. The Role of Sialic Acid in Determining the Survival of Glycoproteins in the Circulation. J. Biol. Chem. 246(5) 1971: 1461–1467.
7 Liu L, et al. The Impact of Glycosylation on the Pharmacokinetics of a TNFR2:Fc Fusion Protein Expressed in Glycoengineered Pichia pastoris. Pharm. Res. 30(3) 2013: 803–812.
8 Liu L. Pharmacokinetics of Monoclonal Antibodies and Fc-fusion Proteins. Protein Cell 19 April 2017: 1–18.
9 Goetze AM, et al. High-Mannose Glycans on the Fc Region of Therapeutic IgG Antibodies Increase Serum Clearance in Humans. Glycobiology 21(7) 2011: 949–959.
10 Oh, DB. Glyco-Engineering Strategies for the Development of Therapeutic Enzymes with Improved Efficacy for the Treatment of Lysosomal Storage Diseases. BMB Rep. 48(8) 2015: 438–444.
11 Ferrara C, et al. Unique Carbohydrate–Carbohydrate Interactions Are Required for High Affinity Binding Between FcγRIII and Antibodies Lacking Core Fucose. Proc. Natl. Acad. Sci. USA 108(31) 2011: 12669–12674.
12 Raju TS. Terminal Sugars of Fc Glycans Influence Antibody Effector Functions of IgGs. Curr. Opin. Immunol. 20(4) 2008: 471–478.
13 Shen, Y. et al. T0001: A Variant of TNFR2-Fc Fusion Protein, Exhibits Improved Fc Effector Functions Through Increased Binding to Membrane-Bound TNFα. PLoS One 12(5) 2017: e0177891.
14 Liu YY. et al. Expression of an Anti-CD3 Single-Chain Immunotoxin with a Truncated Diphtheria Toxin in a Mutant CHO Cell Line. Protein Expr. Purif. 19(2) 2000: 304–311.
15 Liu L. Antibody Glycosylation and Its Impact on the Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharm. Sci. 104(6) 2015: 1866–1884.
16 Wang C, et al. Influence of the Carbohydrate Moiety on the Stability of Glycoproteins. Biochem. 35(23) 1996: 7299–7307.
17 Sinclair AM, Elliott S. Glycoengineering: The Effect of Glycosylation on the Properties of Therapeutic Proteins. J. Pharm. Sci. 94(8) 2005: 1626–1635.
18 Egrie JC and Browne JK. Development and Characterization of Novel Erythropoiesis Stimulating Protein (NESP). Br. J. Cancer 84(Supp 1) 2001: 3–10; doi: 10.1054/bjoc.2001.1746.
19 Mahlangu JN, et al. Phase 1, Randomized, Double-Blind, Placebo-Controlled, Single-Dose Escalation Study of the Recombinant Factor VIIa Variant BAY 86-6150 in Hemophilia. J. Thromb. Haemost. 10(5) 2012: 773–80.
20 Fares F, et al. Designing a Long-Acting Erythropoietin By Fusing Three Carboxyl-Terminal Peptides of Human Chorionic Gonadotropin b Subunit to the N-Terminal and C-Terminal Coding Sequence. Int. J. Cell Biol. 21 August 2011, 1–7; doi: 10.1155/2011/275063.
21 Hart, G. et al. Factor VIIa-CTP, a Novel Long-Acting Coagulation Factor, Displays a Prolonged Hemostatic Effect and Augmented Pharmacokinetics and Pharmacodynamics Following IV and SC Administration in Hemophilic Animal Models. Blood 120(21) 2012: 1114.
22 Chen C, et al. Glycoengineering Approach to Half-Life Extension of Recombinant Biotherapeutics. Bioconjug. Chem. 23(8) 2012: 1524−1533.
23 Wen D, et al. Discovery and Investigation of O-Xylosylation in Engineered Proteins Containing a (GGGGS)n Linker. Anal. Chem. 85(9) 2013: 4805–4812.
24 Zhong X, et al. Pyroglutamate and O-Linked Glycan Determine Functional Production of Anti-IL17A and Anti-IL22 Peptide-Antibody Bispecific Genetic Fusions. J. Biol. Chem. 288, 2013: 1409–1419.
25 Johnson QR, et al. Effects of Branched O-Glycosylation on a Semiflexible Peptide Linker. J. Phys. Chem. B 118(8) 2014: 2050–2057.
26 Butler M, et al. The Choice of Mammalian Cell Host and Possibilities for Glycosylation Engineering. Curr. Opin. Biotechnol. 30, 2014: 107–112.
27 Naso MF, et al. Engineering Host Cell Lines to Reduce Terminal Sialylation of Secreted Antibodies. MAbs 2(5) 2010: 519–527.
28 Zhang M, et al. Enhancing Glycoprotein Sialylation By Targeted Gene Silencing in Mammalian Cells. Biotechnol. Bioeng. 105(6) 2010: 1094–1105.
29 Zhang X, et al. Stable Expression of Human Alpha-2,6-Sialyltransferase in Chinese Hamster Ovary Cells: Functional Consequences for Human Erythropoietin Expression and Bioactivity. Biochim. Biophys. Acta 1425(3) 1998: 441–452.
30 Jeong YT, et al. Enhanced Sialylation of Recombinant Erythropoietin in CHO Cells by Human Glycosyltransferase Expression. J. Microbiol. Biotechnol. 18(12) 2008: 1945–1952.
31 Borys MC, et al. Effects of Culture Conditions on N-glycolylneuraminic Acid (Neu5Gc) Content of a Recombinant Fusion Protein Produced in CHO Cells. Biotechnol. Bioeng. 105(6) 2010: 1048–1057.
32 Rouiller Y, et al. Effect of Hydrocortisone on the Production and Glycosylation of an Fc-Fusion Protein in CHO Cell Cultures. Biotechnol. Prog. 28(3) 2012: 803–813.
33 Okeley NM, et al. Development of Orally Active Inhibitors of Protein and Cellular Fucosylation. Proc. Natl. Acad. Sci. USA 110(14) 2013: 5404–5409.
34 Brühlmann D, et al. Tailoring Recombinant Protein Quality By Rational Media Design. Biotechnol. Prog. 31(3) 2015: 615–629.
35 Kanda Y, et al. Comparison of Biological Activity Among Nonfucosylated Therapeutic IgG1 Antibodies with Three Different N-linked Fc Oligosaccharides: The High-Mannose, Hybrid, and Complex Types. Glycobiology 17(1) 2006: 104–118.
36 Li Y, et al. Heterogeneous Glycoform Separation By Process Chromatography — 1: Monomer Purification and Characterization. J. Chromatogr. A 1404, 2015: 51–59.
37 Rustandi R. Hydrophobic Interaction Chromatography to Analyze Glycoproteins. Methods Mol. Biol. 988, 2013: 211–219.
38 Kishino S, et al. Single-Step Isolation Method for Six Glycoforms of Human α1-Acid Glycoprotein By Hydroxylapatite Chromatography and Study of Their Binding Capacities for Disopyramide. J. Chromatogr. B Biomed. Appl. 703(1–2) 1997: 1–6.
39 Huang BY, et al. Stationary Phases for the Enrichment of Glycoproteins and Glycopeptides. Electrophoresis 35(15) 2014: 2091–2107.
Stefan R. Schmidt is senior vice president of process science and production at Rentschler Biotechnologie GmbH, Erwin-Rentschler-Straße 21, 88471 Laupheim, Germany; 49-7392-701-858; 49-7392-701-300; [email protected]; www.rentschler.de.
You May Also Like





